Abstract
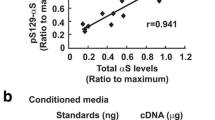
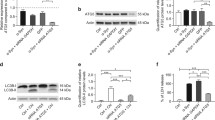
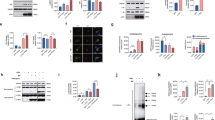
Macroautophagy is a dynamic process whereby cytoplasmic components are initially sequestered within autophagosomes. Recent studies have shown that the autophagosome membrane can selectively recognize ubiquitinated proteins and organelles through interaction with adapter proteins such as p62 and NBR1. Both proteins are structurally similar at the amino acid level, and bind with ubiquitin and ubiquitinated proteins. Although p62 is incorporated into a wide spectrum of pathological inclusions in various neurodegenerative diseases, abnormalities of NBR1 have not been reported in these diseases. Our immunohistochemical examination revealed that the vast majority of Lewy bodies (LBs) in Parkinson’s disease and dementia with LBs (DLB) as well as of glial cytoplasmic inclusions in multiple system atrophy (MSA) were positive for NBR1. Neuronal and glial inclusions in tauopathies and TAR DNA-binding protein of 43 kDa proteinopathies were rarely immunolabeled, or were unstained. Using cultured cells bearing LB-like inclusions, formation of α-synuclein aggregates was repressed in cells with NBR1 knockdown. Immunoblot analysis showed that the level of NBR1 was significantly increased by 2.5-fold in MSA, but not in DLB. These findings suggest that NBR1 is involved in the formation of cytoplasmic inclusions in α-synucleinopathy.







Similar content being viewed by others
References
Arai T, Nonaka T, Hasegawa M et al (2003) Neuronal and glial inclusions in frontotemporal dementia with or without motor neuron disease are immunopositive for p62. Neurosci Lett 342:41–44
Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82:239–259
Braak H, Del Tredici K, Rub Ü et al (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24:197–211
Campbell IG, Nicolai HM, Foulkes WD et al (1994) A novel gene encoding a B-box protein within the BRCA1 region at 17q21.1. Hum Mol Genet 3:589–594
Caughey B, Lansbury PT (2003) Protofibrils, pores, fibrils, and neurodegeneration: separating the responsible protein aggregates from the innocent bystanders. Annu Rev Neurosci 26:267–298
Chambers JA, Solomon E (1996) Isolation of the murine Nbr1 gene adjacent to the murine Brca1 gene. Genomics 38:305–313
Crews L, Spencer B, Desplats P et al (2010) Selective molecular alterations in the autophagy pathway in patients with Lewy body disease and in models of alpha-synucleinopathy. PLoS ONE 5:e9313
D’Agostino C, Nogalska A, Cacciottolo M et al (2011) Abnormalities of NBR1, a novel autophagy-associated protein, in muscle fibers of sporadic inclusion-body myositis. Acta Neuropathol 122:627–636
Engelender S, Kaminsky Z, Guo X et al (1999) Synphilin-1 associates with alpha-synuclein and promotes the formation of cytosolic inclusions. Nat Genet 22:110–114
Higashi S, Moore DJ, Minegishi M et al (2011) Localization of MAP1-LC3 in vulnerable neurons and Lewy bodies in brains of patients with dementia with Lewy bodies. J Neuropathol Exp Neurol 70:264–280
Hocking LJ, Lucas GJ, Daroszewska A et al (2002) Domain-specific mutations in sequestosome 1 (SQSTM1) cause familial and sporadic Paget’s disease. Hum Mol Genet 11:2735–2739
Itakura E, Mizushima N (2011) p62 targeting to the autophagosome formation site requires self-oligomerization but not LC3 binding. J Cell Biol 192:17–27
Johansen T, Lamark T (2011) Selective autophagy mediated by autophagic adapter proteins. Autophagy 7:279–296
Johnson-Pais TL, Wisdom JH, Weldon KS et al (2003) Three novel mutations in SQSTM1 identified in familial Paget’s disease of bone. J Bone Miner Res 18:1748–1753
Kabeya Y, Mizushima N, Ueno T et al (2000) LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J 19:5720–5728
Kawaguchi Y, Kovacs JJ, McLaurin A et al (2003) The deacetylase HDAC6 regulates aggresome formation and cell viability in response to misfolded protein stress. Cell 115:727–738
Kirkin V, Lamark T, Sou YS et al (2009) A role for NBR1 in autophagosomal degradation of ubiquitinated substrates. Mol Cell 33:505–516
Komatsu M, Waguri S, Koike M et al (2007) Homeostatic levels of p62 control cytoplasmic inclusion body formation in autophagy-deficient mice. Cell 131:1149–1163
Kuusisto E, Kauppinen T, Alafuzoff I (2008) Use of p62/SQSTM1 antibodies for neuropathological diagnosis. Neuropathol Appl Neurobiol 34:169–180
Kuusisto E, Salminen A, Alafuzoff I (2001) Ubiquitin-binding protein p62 is present in neuronal and glial inclusions in human tauopathies and synucleinopathies. NeuroReport 12:2085–2090
Lange S, Xiang F, Yakovenko A et al (2005) The kinase domain of titin controls muscle gene expression and protein turnover. Science 308:1599–1603
Laurin N, Brown JP, Morissette J, Raymond V (2002) Recurrent mutation of the gene encoding sequestosome 1 (SQSTM1/p62) in Paget disease of bone. Am J Hum Genet 70:1582–1588
Lee BR, Kamitani T (2011) Improved immunodetection of endogenous alpha-synuclein. PLoS ONE 6:e23939
Lippa CF, Fujiwara H, Mann DM et al (1998) Lewy bodies contain altered alpha-synuclein in brains of many familial Alzheimer’s disease patients with mutations in presenilin and amyloid precursor protein genes. Am J Pathol 153:1365–1370
Mackenzie IR, Neumann M, Baborie A et al (2011) A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol 122:111–113
Mardakheh FK, Yekezare M, Machesky LM, Heath JK (2009) Spred2 interaction with the late endosomal protein NBR1 down-regulates fibroblast growth factor receptor signaling. J Cell Biol 187:265–277
McKeith IG, Dickson DW, Lowe J et al (2005) Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 65:1863–1872
Miki Y, Mori F, Tanji K et al (2011) Accumulation of histone deacetylase 6, an aggresome-related protein, is specific to Lewy bodies and glial cytoplasmic inclusions. Neuropathology 31:561–568
Nakano T, Nakaso K, Nakashima K, Ohama E (2004) Expression of ubiquitin-binding protein p62 in ubiquitin-immunoreactive intraneuronal inclusions in amyotrophic lateral sclerosis with dementia: analysis of five autopsy cases with broad clinicopathological spectrum. Acta Neuropathol 107:359–364
Nishie M, Mori F, Fujiwara H et al (2004) Accumulation of phosphorylated alpha-synuclein in the brain and peripheral ganglia of patients with multiple system atrophy. Acta Neuropathol 107:292–298
Pan T, Kondo S, Le W, Jankovic J (2008) The role of autophagy-lysosome pathway in neurodegeneration associated with Parkinson’s disease. Brain 131:1969–1978
Pandey UB, Nie Z, Batlevi Y et al (2007) HDAC6 rescues neurodegeneration and provides an essential link between autophagy and the UPS. Nature 447:859–863
Stumptner C, Heid H, Fuchsbichler A et al (1999) Analysis of intracytoplasmic hyaline bodies in a hepatocellular carcinoma. Demonstration of p62 as major constituent. Am J Pathol 154:1701–1710
Svenning S, Lamark T, Krause K, Johansen T (2011) Plant NBR1 is a selective autophagy substrate and a functional hybrid of the mammalian autophagic adapters NBR1 and p62/SQSTM1. Autophagy 7:993–1010
Tanji K, Kamitani T, Mori F et al (2010) TRIM9, a novel brain-specific E3 ubiquitin ligase, is repressed in the brain of Parkinson’s disease and dementia with Lewy bodies. Neurobiol Dis 38:210–218
Tanji K, Mori F, Kakita A et al (2011) Alteration of autophagosomal proteins (LC3, GABARAP and GATE-16) in Lewy body disease. Neurobiol Dis 43:690–697
Tanji K, Mori F, Mimura J et al (2010) Proteinase K-resistant alpha-synuclein is deposited in presynapses in human Lewy body disease and A53T alpha-synuclein transgenic mice. Acta Neuropathol 120:145–154
Tanji K, Tanaka T, Mori F et al (2006) NUB1 suppresses the formation of Lewy body-like inclusions by proteasomal degradation of synphilin-1. Am J Pathol 169:553–565
Wakabayashi K, Hayashi S, Kakita A et al (1998) Accumulation of alpha-synuclein/NACP is a cytopathological feature common to Lewy body disease and multiple system atrophy. Acta Neuropathol 96:445–452
Wakabayashi K, Hayashi S, Yoshimoto M et al (2000) NACP/alpha-synuclein-positive filamentous inclusions in astrocytes and oligodendrocytes of Parkinson’s disease brains. Acta Neuropathol (Berl) 99:14–20
Waters S, Marchbank K, Solomon E et al (2009) Interactions with LC3 and polyubiquitin chains link nbr1 to autophagic protein turnover. FEBS Lett 583:1846–1852
Whitehouse C, Chambers J, Howe K et al (2002) NBR1 interacts with fasciculation and elongation protein zeta-1 (FEZ1) and calcium and integrin binding protein (CIB) and shows developmentally restricted expression in the neural tube. Eur J Biochem 269:538–545
Whitehouse CA, Waters S, Marchbank K et al (2010) Neighbor of Brca1 gene (Nbr1) functions as a negative regulator of postnatal osteoblastic bone formation and p38 MAPK activity. Proc Natl Acad Sci USA 107:12913–12918
Yip KH, Feng H, Pavlos NJ et al (2006) p62 ubiquitin binding-associated domain mediated the receptor activator of nuclear factor-kappaB ligand-induced osteoclast formation: a new insight into the pathogenesis of Paget’s disease of bone. Am J Pathol 169:503–514
Zhang HX, Tanji K, Mori F, Wakabayashi K (2008) Epitope mapping of 2E2-D3, a monoclonal antibody directed against human TDP-43. Neurosci Lett 434:170–174
Acknowledgments
This work was supported by Grants-in-Aid 23500425 (K.T.), 23500424 (F.M.), and 20300123 (K.W.) for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology, Japan, a Grant for Hirosaki University Institutional Research (K.W.), the Collaborative Research Project (2011-2209) of the Brain Research Institute, Niigata University (F.M.), Grants-in Aid from the Research Committee for Ataxic Disease, the Ministry of Health, Labor, and Welfare, Japan (K.W.), and an Intramural Research Grant (21B-4) for Neurological and Psychiatric Disorders of NCNP (K.W.). The authors wish to express their gratitude to M. Nakata for her technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Odagiri, S., Tanji, K., Mori, F. et al. Autophagic adapter protein NBR1 is localized in Lewy bodies and glial cytoplasmic inclusions and is involved in aggregate formation in α-synucleinopathy. Acta Neuropathol 124, 173–186 (2012). https://doi.org/10.1007/s00401-012-0975-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-012-0975-7