Abstract
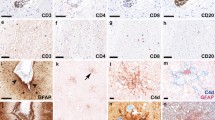
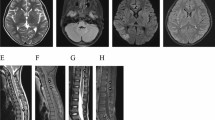
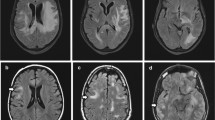
Extensive aquaporin-4 (AQP4) loss without perivascular deposition of either activated complement or immunoglobulins is a characteristic of Baló’s disease. Our aim in this study was to investigate the relationship between astrocytopathy and demyelination in Baló’s disease, focusing on connexins (Cx), which form gap junctions among glial cells and myelin. Autopsied specimens from four cases that provided seven actively demyelinating concentric lesions infiltrated with numerous CD68+ macrophages were immunohistochemically examined for the astrocyte markers glial fibrillary acidic protein (GFAP), AQP4, Cx43, Cx30 and megalencephalic leukoencephalopathy with subcortical cyst 1 (MLC1). Specimens were also stained for oligodendrocyte/myelin markers, namely Cx32, Cx47, myelin-associated glycoprotein (MAG), myelin oligodendrocyte glycoprotein (MOG), oligodendrocyte-specific protein (OSP) and Nogo-A. Serum samples from six patients that had undergone magnetic resonance imaging, confirming a diagnosis of Baló’s disease, were assayed for the presence of anti-Cx43, -Cx32 and -AQP4 antibodies. Despite the presence of numerous GFAP- and MLC1-positive astrocytes, there was a marked decrease in the levels of Cx43, Cx32 and Cx47. At the leading edges, Cx43 and AQP4 were mostly absent despite positive GFAP, MLC1, Cx32, Cx47, MOG, MAG, and OSP immunoreactivity. Of the six Baló’s disease patients, none were positive for anti-Cxs or -AQP4 antibodies. Baló’s disease is characterized by extensive loss of Cxs and AQP4, and a lack of auto-antibodies to Cxs and AQP4. Loss of Cx43 and AQP4 in the presence of other oligodendrocyte/myelin proteins at the leading edges suggests the possibility that auto-antibody-independent astrocytopathy may contribute to disease pathology via the disruption of astrocyte–oligodendrocyte/myelin interactions.







Similar content being viewed by others
References
Aronica E, Gorter JA, Jansen GH, Leenstra S, Yankaya B, Troost D (2001) Expression of connexin 43 and connexin 32 gap-junction proteins in epilepsy-associated brain tumors and in the perilesional epileptic cortex. Acta Neuropathol 101:449–459
Bergoffen J, Scherer SS, Wang S et al (1993) Connexin mutations in X-linked Charcot–Marie-Tooth disease. Science 262:2039–2042
Boor I, Nagtegaal M, Kamphorst W et al (2007) MLC1 is associated with the dystrophin–glycoprotein complex at astrocytic endfeet. Acta Neuropathol 114:403–410
Boor PK, de Groot K, Waisfisz Q et al (2005) MLC1: a novel protein in distal astroglial processes. J Neuropathol Exp Neurol 64:412–419
Brunner C, Lassmann H, Waehneldt TV, Matthieu JM, Linington C (1989) Differential ultrastructural localization of myelin basic protein, myelin/oligodendroglial glycoprotein, and 2′,3′-cyclic nucleotide 3′-phosphodiesterase in the CNS of adult rats. J Neurochem 52:296–304
Garbelli R, Frassoni C, Condorelli DF et al (2011) Expression of connexin 43 in the human epileptic and drug-resistant cerebral cortex. Neurology 76:895–902
John SA, Kondo R, Wang SY, Goldhaber JI, Weiss JN (1999) Connexin-43 hemichannels opened by metabolic inhibition. J Biol Chem 274:236–240
Kamasawa N, Sik A, Morita M et al (2005) Connexin-47 and connexin-32 in gap junctions of oligodendrocyte somata, myelin sheaths, paranodal loops and Schmidt-Lanterman incisures: Implications for ionic homeostasis and potassium siphoning. Neuroscience 136:65–86
Kira J (2011) Astrocytopathy in Baló’s disease. Mult Scler 17:771–779
Kleopa KA, Orthmann JL, Enriquez A, Paul DL, Scherer SS (2004) Unique distribution of gap junction proteins connexin29, connexin32, and connexin47 in oligodendrocytes. Glia 47:346–357
Kuhlmann T, Remington L, Maruschak B, Owens T, Brück W (2007) Nogo-A is a reliable oligodendroglial marker in adult human and mouse CNS and in demyelinated lesions. J Neuropathol Exp Neurol 66:238–246
Lassmann H, Raine CS, Antel J, Prineas JW (1998) Immunopathology of multiple sclerosis: report on an international meeting held at the institute of neurology of the University of Vienna. J Neuroimmunol 86:213–217
Lassmann H (2003) Hypoxia-like tissue injury as a component of multiple sclerosis lesions. J Neurol Sci 206:187–191
Loddenkemper T, Grote K, Evers S, Oelerich M, Stögbauer F (2002) Neurological manifestations of the oculodentodigital dysplasia syndrome. J Neurol 249:584–595
Lucchinetti C, Brück W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H (2000) Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol 47:707–717
Lucchinetti C (2007) Multiple sclerosis pathology during early and late disease phases: pathogenic and clinical relevance. In: Zhang J (ed) Immune regulation and immunotherapy in autoimmune disease. Springer, New York, pp 214–264
Lutz SE, Zhao Y, Gulinello M, Lee SC, Raine CS, Brosnan CF (2009) Deletion of astrocyte connexins 43 and 30 leads to a dysmyelinating phenotype and hippocampal CA1 vacuolation. J Neurosci 29:7743–7752
Magnotti LM, Goodenough DA, Paul DL (2011) Deletion of oligodendrocyte Cx32 and astrocyte Cx43 causes white matter vacuolation, astrocyte loss and early mortality. Glia 59:1064–1074
Matsuoka T, Matsushita T, Kawano Y et al (2007) Heterogeneity of aquaporin-4 autoimmunity and spinal cord lesions in multiple sclerosis in Japanese. Brain 130:1206–1223
Matsuoka T, Suzuki SO, Iwaki T, Tabira T, Ordinario AT, Kira J (2010) Aquaporin-4 astrocytopathy in Baló’s disease. Acta Neuropathol 120:651–660
Matsuoka T, Suzuki SO, Suenaga T, Iwaki T, Kira J (2011) Reappraisal of aquaporin-4 astrocytopathy in Asian neuromyelitis optica and multiple sclerosis patients. Brain Pathol. doi:10.1111/j.1750-3639.2011.00475.x
Matsushita T, Isobe N, Matsuoka T et al (2009) Aquaporin-4 autoimmune syndrome and anti-aquaporin-4 antibody-negative opticospinal multiple sclerosis in Japanese. Mult Scler 15:834–847
Menichella DM, Goodenough DA, Sirkowski E, Scherer SS, Paul DL (2003) Connexins are critical for normal myelination in the CNS. J Neurosci 23:5963–5973
Misu T, Fujihara K, Kakita A et al (2007) Loss of aquaporin 4 in lesions of neuromyelitis optica: distinction from multiple sclerosis. Brain 130:1224–1234
Miyata K, Itoyama Y, Kobayashi T, Yasumori K, Goto I (1990) A case of demyelinating disease showing a peculiar honeycomb-like and lamellar structure on magnetic resonance imaging. Rinsho Shinkeigaku 30:402–406
Morita K, Sasaki H, Fujimoto K, Furuse M, Tsukita S (1999) Claudin-11/OSP-based tight junctions of myelin sheaths in brain and Sertoli cells in testis. J Cell Biol 145:579–588
Nagy JI, Patel D, Ochalski PA, Stelmack GL (1999) Connexin30 in rodent, cat and human brain: selective expression in gray matter astrocytes, co-localization with connexin43 at gap junctions and late developmental appearance. Neuroscience 88:447–468
Nakagawa S, Gong XQ, Maeda S et al (2011) Asparagine 175 of connexin32 is a critical residue for docking and forming functional heterotypic gap junction channels with connexin26. J Biol Chem 286:19672–19681
Nakase T, Yoshida Y, Nagata K (2006) Enhanced connexin 43 immunoreactivity in penumbral areas in the human brain following ischemia. Glia 54:369–375
Orthmann-Murphy JL, Abrams CK, Scherer SS (2008) Gap junctions couple astrocytes and oligodendrocytes. J Mol Neurosci 35:101–116
Orthmann-Murphy JL, Salsano E, Abrams CK et al (2009) Hereditary spastic paraplegia is a novel phenotype for GJA12/GJC2 mutations. Brain 132:426–438
Parratt JD, Prineas JW (2010) Neuromyelitis optica: a demyelinating disease characterized by acute destruction and regeneration of perivascular astrocytes. Mult Scler 16:1156–1172
Paznekas WA, Boyadjiev SA, Shapiro R et al (2003) Connexin 43 (GJA1) mutations cause the pleiotropic phenotype of oculodentodigital dysplasia. Am J Hum Genet 72:408–418
Roemer SF, Parisi JE, Lennon VA et al (2007) Pattern-specific loss of aquaporin-4 immunoreactivity distinguishes neuromyelitis optica from multiple sclerosis. Brain 130:1194–1205
Sargiannidou I, Ahn M, Enriquez A et al (2008) Human oligodendrocytes express Cx31.3: function and interactions with Cx32 mutants. Neurobiol Dis 30:221–233
Sharma R, Fischer MT, Bauer J et al (2010) Inflammation induced by innate immunity in the central nervous system leads to primary astrocyte dysfunction followed by demyelination. Acta Neuropathol 120:223–236
Srinivasan J, Leventer RJ, Kornberg AJ, Dahl HH, Ryan MM (2008) Central nervous system signs in X-Linked Charcot–Marie-Tooth disease after hyperventilation. Pediatr Neurol 38:293–295
Stadelmann C, Ludwin S, Tabira T et al (2005) Tissue preconditioning may explain concentric lesions in Baló’s type of multiple sclerosis. Brain 128:979–987
Taylor RA, Simon EM, Marks HG, Scherer SS (2003) The CNS phenotype of X-linked Charcot–Marie-Tooth disease: More than a peripheral problem. Neurology 61:1475–1478
Uhlenberg B, Schuelke M, Ruschendorf F et al (2004) Mutations in the gene encoding gap junction protein alpha 12 (Connexin 46.6) cause PelizaeusMerzbacher-like disease. Am J Hum Genet 75:251–260
Wang C, Zhang KN, Wu XM et al (2008) Baló’s disease showing benign clinical course and co-existence with multiple sclerosis-like lesions in Chinese. Mult Scler 14:418–424
Yao DL, Webster HD, Hudson LD et al (1994) Concentric sclerosis (Baló): morphometric and in situ hybridization study of lesions in six patients. Ann Neurol 35:18–30
Acknowledgments
This work was supported in part by a Health and Labour Sciences Research Grant on Intractable Diseases (H22-Nanchi-Ippan-130 and H23- Nanchi-Ippan-017) from the Ministry of Health, Labour, and Welfare, Japan, by a Scientific Research B Grant (No. 22390178) and a Challenging Exploratory Research Grant (No. 23659459) from the Ministry of Education, Culture, Sports, Science, and Technology (Japan), by the Kaibara Morikazu Medical Science Promotion Foundation (Japan), and by the Japanese Multiple Sclerosis Society. We thank Professor Artemio T. Ordinario, Department of Neurology and Psychiatry, University of Santo Tomas (Philippines), for providing the Baló’s disease samples, and Ms. Sachiko Koyama and Mr. Takaaki Kanemaru, Department of Neuropathology and Morphology Core Unit, Kyushu University (Japan), for their excellent technical assistance.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
.
401_2012_972_MOESM2_ESM.pdf
Suppl. Fig. 1 Double immunofluorescence staining for GFAP and astrocytic Cxs in a case of myasthenia gravis (MG) (n = 1 case). Punctate staining for Cx43 is distributed in astrocytic cell bodies and processes in the cerebral white matter (a, arrowheads), glial limiting membrane (b, arrow) and perivascular foot processes in the cerebral white matter (c, arrowheads). Cx30 is diffusely expressed in the gray matter of the pons (d). Merged image shows punctate staining for Cx30 frequently abutting from GFAP-immunopositive astrocytic processes. Arrowheads indicate neurons in the gray matter (d). Scale bar: 10 μm (a), 50 μm (b, d), 20 μm (c) (PDF 391 kb)
401_2012_972_MOESM3_ESM.pdf
Suppl. Fig. 2 Double immunofluorescence staining for Cxs and other myelin markers in cases of Pick’s disease (a, b) and myasthenia gravis (MG) (c, d, e) (n = 2 cases). In the spinal cord, Cx32 is localized to the outer layer of myelin sheaths adjacent to GFAP-immunopositive astrocytic processes (a). MAG is localized along the surface of neurofilament-immunopositive axons (b). Immunostaining for Cx32 shows patchy expression along MBP-immunopositive transverse pontine fibers (c). Cx47 is typically expressed around intrafascicular oligodendrocytes in the MBP-immunopositive myelin fibers of the cerebral white matter (d, arrowheads). Double staining for Cx43 and Cx47 shows partial juxtaposition or colocalization suggestive of GJ plaque formation around intrafascicular oligodendrocytes (lower insets) (e). Scale bar: 10 μm (a, d, e), 20 μm (b, c) (PDF 331 kb)
401_2012_972_MOESM4_ESM.pdf
Suppl. Fig. 3 Astrocytic features in cases with other neurological disorders. (a–f) Subacute cerebral infarction. KB staining shows diffuse myelin pallor across the whole area of the lesion (a), but well preserved myelin in the surrounding area (a, upper). CD68 immunostaining shows massive infiltration of macrophages (b). GFAP-positive astrocytes are almost absent across the lesion (c), whereas many reactive astrocytes appear in the surrounding area (c, upper). (d–f) Higher magnification of the lesion (corresponding to Suppl. Fig. 3c, arrowhead). Immunoreactivities for AQP4, MLC1 and Cx43 in the perivascular foot processes are mostly preserved. (g–r) Cx43, Cx30, Cx32 and Cx47 expression in astrogliosis. (g–l) A case of limbic encephalitis. GFAP- (g) and MLC1-positive (h) gemistocytes exhibit membrane staining for Cx43 (i). MLC1 is intensely expressed in the perivascular foot processes and weakly expressed in the cytoplasm of gemistocytes. Immunoreactivity for Cx30 is not evident in gemistocytes (j). Loss of Cx47 and Cx32 are not observed in this astrogliotic lesion (k, l). Arrows indicate the gemistocytes (k, l). (m–r) A case of spastic paraplegia type 2 (SPG2). GFAP- (m) and MLC1-positive (n) reactive astrocytes in a case with chronic fibrillary gliosis show upregulation of Cx43 (o). MLC1 is intensely expressed in perivascular foot processes, and weakly expressed in glial fibers. Immunoreactivity for Cx30 is not evident in this lesion (p), whereas Cx47 and Cx32 expression is preserved (q, r). Scale bar: 200 μm (a–c), 50 μm (d–i), 20 μm (j, m–r), 10 μm (k, l) (PDF 1173 kb)
401_2012_972_MOESM5_ESM.pdf
Suppl. Fig. 4 Measurement of anti-Cx43 and anti-Cx32 antibodies in the sera of patients with Baló’s disease. (a–c) Immunostaining of HEK-293 cells transfected with a GFP-Cx43 fusion protein expression vector. The expressed Cx43-GFP is mainly localized to the plasma membrane (green) and forms gap junction channels at cell–cell interfaces (arrows) with some punctate intracellular distribution. (b) Positive control using an anti-Cx43 antibody. Immunostaining with the anti-Cx43 antibody shows the most intense fluorescence in the region of the gap junction (red). (c) Serum from a patient with Baló’s disease is negative for auto-antibodies against Cx43. (d, e) Immunostaining of HEK-293 cells transfected with a Cx32-GFP fusion protein expression vector. (d) Positive control using an anti-Cx32 antibody. Immunostaining with the anti-Cx32 antibody shows intense fluorescence at cell–cell interfaces (red). (e) Serum from a patient with Baló’s disease is negative for auto-antibodies against Cx32. Scale bar: 10 μm (a–e). DIC: differential interference contrast. (PDF 260 kb)
Rights and permissions
About this article
Cite this article
Masaki, K., Suzuki, S.O., Matsushita, T. et al. Extensive loss of connexins in Baló’s disease: evidence for an auto-antibody-independent astrocytopathy via impaired astrocyte–oligodendrocyte/myelin interaction. Acta Neuropathol 123, 887–900 (2012). https://doi.org/10.1007/s00401-012-0972-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-012-0972-x