Abstract
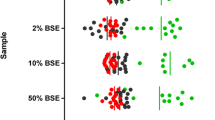
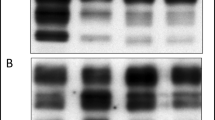
The continuous monitoring of bovine spongiform encephalopathy (BSE) cases is an integral component of European research and surveillance programmes, to ensure that any changes in the presentation of transmissible spongiform encephalopathies (TSE) in cattle can be detected and defined. Monitoring is generally limited to the brainstem at the level of the obex, for reasons of practicality, safety and cost. Demonstration of disease-specific prion protein (PrPd) by immunohistochemistry is currently the most widely used confirmatory tool for both active and passive surveillance. This study assessed PrPd immunostaining in the brainstems (obex) of cattle with BSE in the UK and Italy. Immunoreactivity ‘profiles’ were created for each case based on the nature of the immunostaining, its relative intensity and precise neuroanatomical location. This study compares the obex immunostaining patterns of Italian cases (only active surveillance) and two UK groups (both active and passive surveillance). The neuroanatomical distribution and relative intensity of PrPd was highly reproducible in all cases. The overall staining intensity varied widely but was generally stronger in the active than in the passive surveillance populations. The conclusion to be drawn from this comparative study is that the pattern of immunopathology in these routine screening samples for BSE diagnosis and surveillance is the same in the UK and Italy, whether or not the animal was displaying typical, or indeed any, clinical signs at the time of sampling. This indicates that the current confirmatory diagnostic strategy remains appropriate for active surveillance applications.





Similar content being viewed by others
References
Biacabe A-G, Laplanche J-L, Ryder S, Baron T (2004) Distinct molecular phenotypes in bovine prion diseases. EMBO Rep 2004:1–5
De Bosschere H, Roels S, Vanopdenbosch E (2004) Atypical case of bovine spongiform encephalopathy in an East-Flemish cow. J Appl Res Vet Med 2:52–54
Casalone C, Zanusso GL, Acutis PL, Ferrari S, Capucci L, Tagliavini F, Monaco S, Caramelli M (2004) Identification of a second bovine amyloidotic spongiform encephalopathy: molecular similarities with sporadic Creutzfeldt-Jakob disease. Proc Natl Acad Sci USA 101:3065–3070
Clark WW, Hourrigan JL, Hadlow WJ (1995) Encephalopathy in cattle experimentally infected with the scrapie agent. Am J Vet Res 56:606–612
Cutlip RC, Miller JM, Race RE, Jenny AL, Katz JB, Lehmkuhl HD, DeBey BM, Robinson MM (1994) Intracerebral transmission of scrapie to cattle. J Infect Dis 169:814–820
European Commission (1996) European Union Council Regulation 716/1996/EC. Official J L099:14
European Commission (2001) European Union Council Regulation 999/2001/EC. Official J L147:1
European Commission (2002). European Union Council Regulation 270/2002/EC. Official J L45:4
European Commission (2004) Report on the monitoring and testing of Ruminants for the presence of transmissible spongiform encephalopathy (TSE) in the EU in 2003, including the results of the survey of prion protein genotypes in sheep breeds. http://www.europa.eu.int/comm/food/food/biosafety/bse/annual_reps_en.htm
Hoinville LJ, Wilesmith JW, Richards MS (1995) An investigation of risk factors for cases of bovine spongiform encephalopathy born after the introduction of the ‘feed ban’. Vet Rec 136:312–318
Jeffrey M, Martin S, Gonzalez L (2003) Cell-associated variants of disease-specific prion protein immunolabelling are found in different sources of sheep transmissible spongiform encephalopathy. J Gen Virol 84:1033–1145
Robinson MM, Hadlow WJ, Knowles DP, Huff TP, Lacy PA, Marsh RF, Gorham JR (1995) Experimental infection of cattle with the agents of transmissible mink encephalopathy and scrapie. J Comp Pathol 113:241–251
Ryder SJ, Hawkins SAC, Konold T, Wells GAH (2002) Challenge of cattle with two current sources of British ovine scrapie results in two different disease presentations. In: Proceedings of the international conference on transmissible spongiform encephalopathies, 15–18 September 2002, Edinburgh, p 22
Simmons MM, Harris P, Jeffrey M, Meek S, Blamire IWH, Wells GAH (1996) BSE in Great Britain: consistency of the neurohistological findings in two random annual samples of clinically suspect cases. Vet Rec 146:391–395
Terry LA, Marsh SA, Ryder SJ, Hawkins SAC, Wells GAH, Spencer YI (2003) Detection of disease-specific PrP in the distal ileum of cattle exposed orally to the agent of bovine spongiform encephalopathy. Vet Rec 152:387–92
Wells GAH, Hancock RD, Cooley WA, Richards MS, Higgins RJ, David GP (1989) Bovine spongiform encephalopathy: diagnostic significance of vacuolar change in selected nuclei of the medulla oblongata. Vet Rec 125:521–524
Wells GAH, Hawkins SAC, Green RB, Austin AR, Dexter I, Spencer YI, Chaplin MJ, Stack MJ, Dawson M (1998) Preliminary observations on the pathogenesis of experimental bovine spongiform encephalopathy (BSE): an update. Vet Rec 142:103–106
Wells GAH, Wilesmith JW (1995) The neuropathology and epidemiology of bovine spongiform encephalopathy. Brain Pathol 5:91–103
Yamakawa Y, Hagiwara K, Nohtomi K, Nakamura Y, Nishijima M, Higuchi Y, Sato Y, Sata T (2003) Expert Committee for BSE diagnosis, Ministry of Health, Labour and Welfare of Japan. Atypical proteinase K-resistant prion protein (PrPres) observed an apparently healthy 23-month-old Holstein steer. Jpn J Infect Dis 56:221–222
Acknowledgements
The authors wish to thank the staff of the Pathology Department, VLA-Weybridge, and the CEA, IZS Turin for their excellent technical assistance. This work was funded by Defra (Project SE 0225 to MMS) and the Italian Ministry of Health (IZS PLV 001/01 to CC).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Casalone, C., Caramelli, M., Crescio, M.I. et al. BSE immunohistochemical patterns in the brainstem: a comparison between UK and Italian cases. Acta Neuropathol 111, 444–449 (2006). https://doi.org/10.1007/s00401-005-0012-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-005-0012-1