Abstract
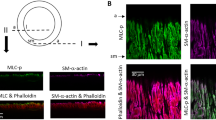
We have characterised the blood vessels found in normal cerebral vasculature and in arteriovenous malformations (AVMs), based on the expression of smooth muscle cell (SMC)-specific proteins. The marker proteins used were smooth muscle α-actin and four myosin heavy chain isoforms (SM1, SM2, SMemb and NMHC-A). Specimens of AVM obtained during surgery, and normal cerebral vessels from autopsy cases were studied immunohistochemically and compared. The arterial components of AVM contained an abundance of SMCs of the contractile phenotype, which were positive for α-actin, SM1 and SM2, but not for SMemb and NMHC-A. These components showed the same staining pattern as mature normal arteries. Two different types of abnormal veins were found in the AVM specimens: large veins with a thick and fibrous wall (so-called 'arterialised' veins) and intraparenchymal thin-walled sinusoidal veins. The former expressed α-actin, SM1, SM2, and SMemb, the latter expressed α-actin, SM1, and SM2. These marker expression patterns resembled those of normal cerebral arteries, and the results were compatible with arterialisation of the cerebral veins caused by arteriovenous shunting. However, the expression of SMemb was found only in the arterialised type of veins, not in the sinusoidal type or the arteries that had sustained abnormal blood flow in the AVMs. The thick-walled veins in the AVMs showed the same staining pattern as normal veins of dural plexus origin (large subarachnoid veins and dural sinuses). It is therefore possible to assume that they originated from the dural plexus, and extended into the brain during the formation of AVMs.





Similar content being viewed by others
References
Aikawa M, Sivam PN, Kuro-o M, Kimura K, Nakahara K, Takewaki S, Ueda M, Yamaguchi H, Yazaki Y, Periasamy M, Nagai R (1993) Human smooth muscle myosin heavy chain isoforms as molecular markers for vascular development and atherosclerosis. Circ Res 73:1000–1012
Cliff WJ (1967) The aortic tunica media in growing rats studied with the electron microscope. Lab Invest 17:599–615
Cormack DH (1993) Essential histology. Lippincott, Philadelphia
Darby I, Skalli O, Gabbiani G (1990) α-Smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest 63:21–29
Eddinger TJ, Murphy RA (1991) Developmental changes in actin and myosin heavy chain isoform expression in smooth muscle. Arch Biochem Biophys 284:232–237
Fatigati V, Murphy RA (1984) Actin and tropomyosin variants in smooth muscles. Dependence on tissue type. J Biol Chem 259:14383–14388
Garretson HD (1996) Intracranial arteriovenous malformations. In: Wilkins RH, Rengachary SS (eds) Neurosurgery, 2nd edn, vol 2. McGraw-Hill, New York, pp 2433–2442
Gerrity RG, Cliff WJ (1975) The aortic tunica media of the developing rat. I. Quantitative stereologic and biochemical analysis. Lab Invest 32:585–600
Hoya K, Asai A, Sasaki T, Kimura K, Kirino T (2001) Expression of smooth muscle proteins in cavernous and arteriovenous malformations. Acta Neuropathol 102:257–263
Jellinger K (1986) Vascular malformations of the central nervous system: a morphological overview. Neurosurg Rev 9:177–216
Joyce NC, Haire MF, Palade GE (1985) Contractile proteins in pericytes. II. Immunocytochemical evidence for the presence of two isomyosins in graded concentrations. J Cell Biol 100:1387–1395
Junqueira LC, Caneiro J, Kelley RO (1992) Basic histology, 7th edn. Prentice-Hall International, London
Kawamoto S, Adelstein RS (1987) Characterization of myosin heavy chains in cultured aorta smooth muscle cells. A comparative study. J Biol Chem 262:7282–7288
Kee DB, Wood JH (1987) Influence of blood rheology on cerebral circulation. In: Wood JH (ed) Cerebral blood flow. McGraw-Hill, New York, pp 173–185
Kocher O, Skalli O, Bloom WS, Gabbiani G (1984) Cytoskelton of rat aortic smooth muscle cells. Normal conditions and experimental intimal thickening. Lab Invest 50:645–652
Kocher O, Skalli O, Cerutti D, Gabbiani F, Gabbiani G (1985) Cytoskeletal features of rat aortic cells during development. An electron microscopic, immunohistochemical, and biochemical study. Circ Res 56:829–838
Kockx MM, Cambier BA, Bortier HE, De Meyer GR, Van Cauwelaert PA (1992) The modulation of smooth muscle cell phenotype is an early event in human aorto-coronary saphenous vein grafts. Virchows Arch [A] 420:155–162
Kuro-o M, Nagai R, Tsuchimochi H, Katoh H, Yazaki Y, Ohkubo A, Takaku F (1989) Developmentally regulated expression of vascular smooth muscle myosin heavy chain isoforms. J Biol Chem 264:18272–18275
Kuro-o M, Nagai R, Nakahara K, Katoh H, Tsai RC, Tsuchimochi H, Yazaki Y, Ohkubo A, Takaku F (1991) cDNA cloning of a myosin heavy chain isoform in embryonic smooth muscle and its expression during vascular development and in arteriosclerosis. J Biol Chem 266:3768–3773
Lagos JC (1977) Congenital aneurysms and arteriovenous malformations. In: Vinken PJ, Bruyn GW (eds) Handbook of clinical neurology; congenital malformations of the brain and skull, vol 2. North Holland, Amsterdam, pp 137–209
Mandybur TI, Nazek M (1990) Cerebral arteriovenous malformations: a detailed morphological and immunohistochemical study using actin. Arch Pathol Lab Med 114:970–973
Martin N, Vinters N (1990) Pathology and grading of intracranial vascular formations. In: Barrow DL (ed) Intracranial vascular malformations. American Association of Neurological Surgeons, Park Ridge, pp 1–30
Meng JS, Okeda R (2001) Histopathological structure of the pial arteriovenous malformation in adults: observation by reconstruction of serial sections of four surgical specimens. Acta Neuropathol 102:63–68
Mullan S, Mojtahedi S, Johnson DL, MacDonald RL (1996) Embryological basis of some aspects of cerebral vascular fistulas and malformations. J Neurosurg 85:1–8
Nehls V, Drenckhahn D (1991) Heterogeneity of microvascular pericytes for smooth muscle type alpha-actin. J Cell Biol 113:147–154
Nehls V, Drenckhahn D (1993) The versatility of microvascular pericytes: from mesenchyme to smooth muscle? Histochemistry 99:1–12
Okai-Matsuo Y, Takano-Omuro H, Toyo-oka T, Sugimoto T (1991) A novel myosin heavy chain isoform in vascular smooth muscle. Biochem Biophys Res Commun 176:1365–1370
Owens GK, Thompson MM (1986) Developmental changes in isoactin expression in rat aortic smooth muscle cells in vivo. Relationship between growth and cytodifferentiation. J Biol Chem 261:13373–13380
Padget DH (1956) The cranial venous system in man in reference to development, adult configuration, and relation to the arteries. Am J Anat 98:307–356
Quist WC, Haudenschild CC, LoGerfo FW (1992) Qualitative microscopy of implanted vein grafts. Effects of graft integrity on morphological fate. J Thorac Cardiovasc Surg 103:671–677
Ross R, Wight TN, Strandness E, Thiele B (1984) Human atherosclerosis. I. Cell constitution and characteristics of advanced lesions of the superficial femoral artery. Am J Pathol 114:79–93
Sappino AP, Schürch W, Gabbiani G (1990) Biology of disease. Differentiation repertoire of fibroblastic cells: expression of cytoskeletal proteins as marker of phenotypic modulations. Lab Invest 63:144–161
Stehbens WE (1972) Pathology of the cerebral blood vessels. Mosby, St. Louis, pp 471–558
Stevens A, Lowe JS (1997) Human histology, 2nd edn. Mosby, London
Verbeek MM, Otte-Höller I, Wesseling P, Ruiter DJ, Waal RMW de (1994) Induction of α-smooth muscle actin expression in cultured human brain pericytes by transforming growth factor-β1. Am J Pathol 144:372–382
Warkany J, Lemire RJ (1984) Arteriovenous malformations of the brain. A teratologic challenge. Teratology 29:333–353
Yasargil MG (1987) Pathological considerations. In: Yasargil MG (ed) Microneurosurgery, IIIA. Thieme, Stuttgart, pp 49–211
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hoya, K., Asai, A., Sasaki, T. et al. Expression of myosin heavy chain isoforms by smooth muscle cells in cerebral arteriovenous malformations. Acta Neuropathol 105, 455–461 (2003). https://doi.org/10.1007/s00401-002-0665-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-002-0665-y