Abstract
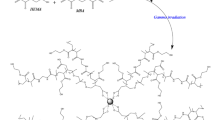
A novel method was developed to synthesize air-stable zerovalent iron nanoparticles (hereafter nZVI) utilizing a radiation grafting technique. The nZVI were synthesized by borohydrate reduction of FeCl3 and stabilized on a radiation grafted copolymer matrix. Polyacrylic acid (PAA) grafted non-woven polyethylene/polypropylene (NWPE/PP-g-PAA) was used as the copolymer matrix and Co-60 γ-radiation was applied. The nZVI adsorbed NWPE/PP-g-PAA (hereafter nZVI-Ads-NWP) polymer composites were characterized by X-ray diffraction (XRD), X-ray photoelectron spectroscopy (XPS), vibrational spectroscopy, scanning electron microscopy (SEM), Mössbauer spectroscopy, proton titrations, and zeta potential techniques. The SEM images showed that PAA was properly grafted onto the NWPE/PP fabric during irradiation and that the nZVI were well dispersed and stabilized on the fabric surface. Vibrational spectroscopy showed supplementary evidence for the proper grafting of PAA onto the base polymer and suggested a monodentate configuration as the primary interaction between the carboxylate groups of PAA and the nZVI surface. XRD, XPS, and Mössbauer analyses revealed core zerovalent iron with a shell mainly consisting of iron oxides. The pHZPC and pHIEP values of nZVI–NaCl suspensions were 7.3. Zeta potential and surface charge data were modeled using the 1-pK Stern layer model with two dissimilar sites for electrolyte and proton binding to account for the observed charge asymmetry.
Access this article
We’re sorry, something doesn't seem to be working properly.
Please try refreshing the page. If that doesn't work, please contact support so we can address the problem.











Similar content being viewed by others
References
Fu F, Dionysiou DD, Liu H (2014) J Hazard Mater 267:194–205
Andreas T, Silke K, Yuri K, Aharon G (2009) Ultrason Sonochem 16:617–621
Dror I, Jacov OM, Cortis A, Berkowitz B (2012) Appl Mater Interfaces 4:3416–3423
Satapanajaru T, Chompuchan C, Suntornchot P, Pengthamkeerati P (2011) Desalination 266:218–230
Poursaberi T, Hassanisadi M, Nourmohammadian F (2012) Prog Color Colorants Coat 5:35–40
El-Temsaha YS, Sevcub A, Bobcikova K, Cernik M, Joner EJ (2016) Chemosphere 144:2221–2228
Yang J, Sun H (2015) Water Air Soil Pollut 226:1–15
Yan W, Herzing AA, Kiely CJ, Zhang WX (2010) J Contam Hydrol 118:96–104
Zhang X, Lin S, Lu XQ, Chen ZL (2010) Chem Eng J 163:243–248
Fang Z, Quid X, Huang R, Quid X, Li M (2011) Desalination 280:224–231
Hwang YH, Kim D–G, Shin H-S (2011) J Hazard Mater 185:1513–1521
Khalid AME, Eljamal O, Jribi S, Matsunaga N (2016) Chem Eng J 287:367–380
Noubactep C (2015) Water Res 85:114–123
Sun YP, Li X, Cao J, Zhang W, Wang HP (2006) Adv Colloid Interface Sci 120:47–56
Ponder SM, Darab JG, Mallouk TE (2000) Environ Sci Technol 34:2564–2569
Qui X, Fang Z, Liang B, Gu F, Xu Z (2011) J Hazard Mater 193:70–81
Stefaniuk M, Oleszczuk P, Ok YS (2016) Chem Eng J 287:618–632
Li A, Tai C, Zhan ZS, Wang YW, Zhang QZ, Jiang GB, Hu JT (2007) Environ Sci Technol 41:6841–6846
An Y, Li T, Jin Z, Dong M, Li Q, Wang S (2009) Sci Total Environ 407:5465–5470
Morgada ME, Levy IK, Salomone V, Farías SS, López G, Litter MI (2009) Catal Today 143:261–268
Machado S, Pinto SL, Grosso JP, Nouws HPA, Albergaria JT, Matos CD (2013) Sci Total Environ 445–446:1–8
Liua HB, Chena TH, Changa DY, Chena D, Liua Y, Heb HP, Yuanb P (2012) Mater Chem Phys 133:205–211
Oropeza S, Corea M, Gómez-Yáñez C, Cruz-Rivera JJ, Clemente MEN (2012) Mater Res Bull 47:1478–1485
He F, Zhao DY, Liu JC, Roberts CB (2007) Ind Eng Chem Res 46:29–34
Kassaeea MZ, Motamedia E, Mikhakb A, Rahnemaie R (2011) Chem Eng J 166:490–495
Li L, Fan M, Brown RC, Leeuwen LV (2006) Environ Sci Technol 36:1–13
Barsbay M, Guven O (2013) Polymer 54:4838–4848
Clough RL (2001) Nucl Inst Methods 185:8–33
Spinks JWT, Woods RJ (1990) Ions, excited molecules and free radicals. In: Spinks JWT, Woods RJ (eds) An introduction to radiation chemistry. Wiley, New York, pp 127–177
Cirtiu CM, Raychoudhury T, Ghoshal S, Moores A (2011) Colloids Surf A 390:95–104
Allabaksh MB, Mandal BK, Kesarla MK, Kumar KS, Reddy PS (2010) J Chem Pharm Res 2:67–74
Yuvakkumar R, Elango V, Rajendran V, Kannan N (2011) Digest J Nano Mater Bio-Struct 6:1771–1776
Madhavi V, Prasad TNVKV, Reddy AVB, Reddy BR, Madhavi G (2013) Spectrochim Acta A 116:17–25
Maczka E, Jartych E, Kosmulski M (2014) Colloids Surf A 441:326–330
Ratnayake S, Bandara A, Wijayawardhana RL, Weerasooriya R (2015) Air stable nZVI fabrication method. PGIS Research Congress, 9–10th October, University of Peradeniya, Sri Lanka, pp 22–23
Seah MP, Gilmore IS, Beamson G (1998) Surf Interface Anal 26:642–649
Westall JC (1982) FITEQL: a computer program for determination of chemical equilibrium constants from experimental data, version 2.0. Chemistry (USA) Report no 82-02. Oregon State University, Corvallis, Oregon
Poeter EP, Hill MC (1999) UCODE—a computer code for universal inverse modeling. Comput Geosci 25:457–462
Lützenkirchen J, Preočanin T, Kallay N (2008) Phys Chem Chem Phys 10:4946–4955
Goel NK, Bhardwaj YK, Manoharan R, Kumar V, Dubey KA, Chaudhari CV, Sabharwal S (2009) e-XPRESS Polym Lett 3:268–278
Ibrahim SM, El-Salmawi KM, El-Naggar AA (2006) J Appl Polym Sci 102:3240–3245
Hietala S, Holmberg S, Karjalainen M, Nasman J, Paronen M, Serimaa R, Sundholm F, Vahvaselka S (1997) J Mater Chem 7:721–726
De Ming F, Peng Y, Tian Hu C, Hong Ping H, Ai Hua Y, Kang Min C, Jian Xi Z, Dong L (2010) Chin Sci Bull 55:1092–1099
Machala L, Zboril R, Gedanken A (2007) J Phys Chem B 111:4003–4018
Siskova K, Tucek J, Machala L, Otyepkova E, Filip J, Safarova K, Pechousek J, Zboril R (2012) J Nanopart Res 14:1–13
Ji Y (2014) Colloids Surf A 444:1–8
Parks GA (1965) Chem Rev 65:177–198
Kosmulski M (2009) Adv Colloid Interface Sci 152:14–25
Kallay N, Orbic Z, Golic M, Matijevic E (1991) J Phys Chem 95:7028–7032
Goldberg S, Forster HS, Heick EL (1993) Soil Sci Am J 57:704–708
Heberling F, Thomas P, Trainor TP, Lützenkirchen J, Eng P, Denecke MA, Bosbach D (2011) J Colloid Interface Sci 354:843–857
Lützenkirchen J (1998) Environ Sci Technol 32:3149–3154
Acknowledgments
Sudeera Randenigama is acknowledged for laboratory assistance. The InRC, UoP (Sri Lanka), is acknowledged for SEM facility. RW thanked the National Research Council of Sri Lanka for financial support provided under grant no. NRC-12-130. SR thanked the Sri Lanka Atomic Energy Board and Sri Lanka Gamma Centre for providing the irradiation facilities. Reviewers’ comments enhanced manuscript quality.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Ratnayake, S., Schild, D., Maczka, E. et al. A novel radiation-induced grafting methodology to synthesize stable zerovalent iron nanoparticles at ambient atmospheric conditions. Colloid Polym Sci 294, 1557–1569 (2016). https://doi.org/10.1007/s00396-016-3894-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-016-3894-7