Abstract
The interplay of several non-covalent interaction forces is used as key to supramolecular structures. Combining cationic alkyltrimethylammonium bromide surfactants and the divalent anionic azo dye Acid Red 26 (Ar26) as small building blocks in aqueous solution, electrostatic interactions of the oppositely charged building blocks in combination with hydrophobic effect and π–π interactions play a major role in aggregate formation. Static and dynamic light scattering and small-angle neutron scattering (SANS) revealed different sizes of aggregates in the range of 2 nm ≤ R H ≤ 420 nm depending on surfactant length, concentration and of dye to surfactant loading ratio. A strong relationship of assembly size with surfactant concentration has been found, where initial surfactant monomers and micelles influence the aggregate formation differently. The stability of dye–surfactant aggregates which also shows a dependency on surfactant tail length has been related to ζ-potential measurements. Small-angle neutron scattering elucidated that dye–surfactant aggregates possess cylindrical shapes with different aspect ratios. UV/Vis spectroscopy gave information on the dye–dye π–π stacking geometry and extent, while the thermodynamic parameters for micellization and dye–surfactant binding ΔH, ΔG, and ΔS as well as stoichiometry and binding constant obtained by isothermal titration calorimetry revealed insight into the interplay of interactions.













Similar content being viewed by others
Notes
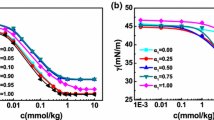
The weight of peak was found to be 15 % for the first peak and 85 % for the second peak.
The weight of peak was found to be 16 % for the first peak and 84 % for the second peak.
The weight of peak was found to be 65 % for the first peak and 35 % for the second peak.
For aggregates with l = 0.7, dimensions of at least 153 nm in length and 4.7 nm in diameter were found whereas aggregates with l = 0.5 were at least 233 nm in length and 5.6 nm in diameter.
References
Imae T, Kamiya R, Ikeda S (1985) J Colloid Interface Sci 108:215–225
Aswal VK (2003) J Phys Chem B 107:13323–13328
Diaz Garcia ME, Sanz-Medel A (1986) Talanta Int J Pure Appl Anal Chem 33:255–264
Ribeiro ACF, Lobo VMM, Valente AJM, Azevedo EFG, Miguel MG, Burrows HD (2004) Colloid Polym Sci 283:2777–283
Klitzing RV, Espert A, Asnacios A, Hellweg T, Colin A, Langevin D (1999) Colloids Surface A 149:131–140
Fitzgerald PA, Davey TW, Warr GG (2005) Langmuir 21:7121–7128
Seliverstova EV, Ibrayev NK, Kudaibergenov SE (2013) Russ J Phys Chem A 87:865–871
Seliverstova EV, Ibrayev NK, Kudaibergenov SE (2013) J Appl Polym Sci 129:289–295
Hu W, Ong WL, Ho GW (2010) Colloids Surf A 358:108–114
Kasture M, Sastry M, Prasad BLV (2010) Chem Phys Lett 484:271–275
Gradzielski M, Kumar S, Mehta SK (2011) J Colloid Interface Sci 360:497–507
Gradzielski M, Wagner NJ, Schweins R, Prévost S, Heunemann P, Hoffmann I (2011) Langmuir 27:4386–4396
Sidhu J, Bloor DM, Couderc-Azouani S, Penfold J, Holzwarth JF, Wyn-Jones E (2004) Langmuir 20:9320–9328
Ganeva D, Faul CFJ, Götz C, Sanderson R (2003) Macromolecules 36:2862–2866
Ganeva D, Antonietti M, Faul CFJ, Sanderson R (2003) Langmuir 19:6561–6565
Wang Y, Kimura K, Dubin PL (2000) Macromolecules 33:3324–3331
Xia J, Zhang H, Rigsbee DR, Dubin PL, Shaikh T (1993) Macromolecules 26:2759–2766
Sudbeck EA, Dubin PL, Curran ME, Skelton I (1991) J Colloid Interface Sci 142:512–517
Dubin PL, Oteri R (1983) J Colloid Interface Sci 95:453–461
Antonietti M, Burger C, Effing J (1995) Adv Mater 7:751–753
Antonietti M, Conrad J, Thünemann A (1994) Macromolecules 27:6007–6011
Thünemann AF, Lochhaas KH (1998) Langmuir 14:6220–6225
Hoffmann I, Farago B, Schweins R, Falus P, Sharp M, Gradzielski M (2013) EPL 104:28001
Antonietti M, Förster S (2003) Adv Mater 15:1323–1333
Wang Y, Han P, Xu H, Wang Z, Zhang X, Kabanov A (2009) Langmuir 26:709–715
Yan Q, Yuan J, Cai Z, Xin Y, Kang Y, Yin Y (2010) J Am Chem Soc 132:9268–9270
Ren Y, Baumgartner T (2012) Dalton Trans 41:7792–7800
Babu SS, Prasanthkumar S, Ajayaghosh A (2012) Angew Chem Int Ed 51:1766–1776
Faul CFJ (2014) Acc Chem Res 47:3428–3438
Faul CFJ, Antonietti M (2002) Chem Eur J 8:2764–2768
Priimagi A, Vapaavuori J, Rodriguez FJ, Faul CFJ, Heino MT, Ikkala O, Kauranen M, Kaivola M (2008) Chem Mater 20:6358–6363
Guan Y, Antonietti M, Faul CFJ (2002) Langmuir 18:5939–5945
Zakrevskyy Y, Stumpe J, Faul CFJ (2006) Adv Mater 18:2133–2136
Franke D, Egger CC, Smarsly B, Faul CFJ, Tiddy GJT (2005) Langmuir 21:2704–2712
Wang Y, Ma N, Wang Z, Zhang X (2007) Angew Chem Int Ed 46:2823–2826
Wang Y, Li W, Wu L (2009) Langmuir 25:13194–13200
Štěpánek M, Škvarla J, Uchman M, Procházka K, Angelov B, Kováčik L, Garamus VM, Mantzaridis C, Pispas S (2012) Soft Matter 8:9412–9417
Minard-Basquin C, Weil T, Hohner A, Rädler JO, Müllen K (2003) J Am Chem Soc 125:5832–5838
Kaper H, Djerdj I, Gross S, Amenitsch H, Antonietti M, Smarsly BM (2015) Phys Chem Chem Phys 17:18138–18145
Škvarla J, Uchman M, Procházka K, Tošner Z, Garamusc VM, Pispas S, Štěpánek M (2014) Colloids Surf A 443:209–215
Uchman M, Gradzielski M, Angelov B, Tošner Z, Oh J, Chang T, Štěpánek M, Procházka K (2013) Macromolecules 46:2712–2181
Uchman M, Štěpánek M, Prévost S, Angelov B, Bednár J, Appavou M-S, Gradzielski M, Procházka K (2012) Macromolecules 45:6471–6480
Hajduová J, Procházka K, Šlouf M, Angelov B, Mountrichas G, Pispas S, Stěpánek M (2012) Langmuir 29:5443–5449
Chiappisi L, Simon M, Gradzielski M (2015) ACS Appl Mater Interfaces 7:6139–6145
Chiappisi L, Gradzielski M (2015) Adv Colloids Interface Sci 220:91–107
Huang J-B, Yan Y, Gao C, Peng Y, Cheng X-H (2013) Colloids Surf A 422:10–18
Zhang T, Liu S, Kurth DG, Faul CFJ (2009) Adv Funct Mater 19:642–652
Zhang T, Spitz C, Antonietti M, Faul CFJ (2005) Chem Eur J 11:1001–1009
Willerich I, Gröhn F (2008) Chem Eur J 14:9112–9116
Willerich I, Ritter H, Gröhn F (2009) J Phys Chem B 113:3339–3354
Willerich I, Li Y, Gröhn F (2010) J Phys Chem B 114:15466–15476
Willerich I, Schindler T, Gröhn F (2011) J Phys Chem B 115:9710–9719
Willerich I, Gröhn F (2011) J Am Chem Soc 133:20341–20356
Faul CFJ, Antonietti M (2003) Adv Mater 15:673–683
Willerich I, Gröhn F (2010) Angew Chem Int Ed 49:8104–8108
Gröhn F (2008) Macromol Chem Phys 209:2295–2301
Bielska M, Sobczyńska A, Prochaska K (2009) Dyes Pigments 80:201–205
Karukstis KK, Savin DA, Koftus CT, DʼAngelo N (1998) J Colloid Interface Sci 203:157–163
Huang J-B, Yan Y, Gao C, Peng Y, Cheng X-H (2013) Colloids Surfaces A 422:10–18
Wang D, Long P, Dong R, Hao J (2012) Langmuir 28:14155–14163
Rudorf S, Rädler JO (2012) J Am Chem Soc 134:11652–11658
Hohner A, Bayer J, Rädler JO (2006) Eur Phys J E 21:41–48
Laiho A, Smarsly BM, Faul CFJ, Ikkala O (2008) Adv Funct Mater 18:1890–1897
Provencher SW (1982) Comput Phys Commun 27:229–242
Glatter O (1977) Acta Phys Austriaca 47:83–102
Glatter O (1977) J Appl Crystallogr 10:415–421
Greaves TL, Dummond C (2008) J Chem Soc Rev 37:1709–1726
Tadros TF (2005) Applied surfactants: principles and applications. VCH, Weinheim
Aswal VK (2003) Barc Newsl 237:37–42
Aswal VK, Goyal PS (2004) Praman – J Phys 63:65–72
Aswal VK, Goyal PS (2002) Chem Phys Lett 357:491–497
Naskar B, Dan A, Ghosh S, Aswal VK, Moulik PS (2012) J Mol Liq 170:1–10
Nasiruddin M, Sarwar A (2006) Fluid Phase Equilib 239:166–171
Iampietro DJ, Brasher LL, Kaler EW, Stradner A, Glatter O (1998) J Phys Chem B 102:3105–3113
Quirion F, Magid LJ (1986) J Phys Chem 90:5435–5441
Goyal PS, Srinivasa K, Dasannacharya BA, Kelkar VK (1991) Physica B 174:192–195
Ghai R, Falconer RJ, Collins BM (2012) J Mol Recognit 25:32–52
Chiad K, Stelzig SH, Gropeanu R, Weil T, Klapper M, Müllen K (2009) Macromolecules 42:7545–7552
Mosquera V, del Río JM, Attwood D, García M, Jones MN, Prieto G, Suarez MJ, Sarmiento F (1998) J Colloid Interface Sci 206:66–76
Lah J, Pohar C, Vesnaver G (2000) J Phys Chem B 104:2522–2526
Helgeson ME, Hodgdon TK, Kaler EW, Wagner NJ (2010) J Colloid Interface Sci 349:1–12
Bouchemal K, Agnely F, Koffi A, Djabourov M, Ponchel G (2010) J Mol Recognit 23:335–342
Courtois J, Berret J-F (2010) Langmuir 26:11750–11758
Acknowledgments
This work is based upon experiments performed at D11 at Institit Laue Langevin, Grenoble France and at the KWS 2 instrument operated by JCNS at the Heinz Maier-Leibnitz Zentrum (MLZ), Garching, Germany. We thank Ralf Schweins (ILL) and Henrich Frielinghaus (MLZ) for help with SANS experiments.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by the Interdisciplinary Center for Molecular Materials (ICMM, University Erlangen-Nürnberg). The authors gratefully acknowledge the financial support provided by Institit Laue Langevin, Grenoble France and by JCS to perform the neutron scattering measurements at ILL and at the Heinz Maier-Leibnitz Zentrum (MLZ), Garching, Germany.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kutz, A., Mariani, G. & Gröhn, F. Ionic dye–surfactant nanoassemblies: interplay of electrostatics, hydrophobic effect, and π–π stacking. Colloid Polym Sci 294, 591–606 (2016). https://doi.org/10.1007/s00396-015-3814-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-015-3814-2