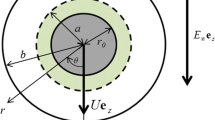
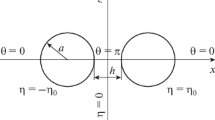
Abstract
Electrophoretic mobilities (EPM) of negatively charged latex spheres were measured as a function of salt type and salt concentration. The measured values of EPM were analyzed using a standard electrokinetic model that includes double layer relaxation and the Poisson–Boltzmann model of diffuse double layer. Calculated values of EPM were in good agreement with experimental data taken in simple 1:1 (KCl) and 1:2 (Na2SO4) electrolyte solutions without using any fit parameters. For 2:1 electrolytes (CaCl2 and MgCl2), however, the magnitude of EPM calculated by the model was higher than the measured values of EPM at higher electrolyte concentrations. The difference between measured and calculated EPM was reduced by assuming the distance of slipping plane x s = 0.25 nm or by assuming the decrease of the magnitude of surface charge density from −0.07 to −0.025 C/m2. These are probably due to the accumulation of divalent counterions in the vicinity of a particle’s surface.




Similar content being viewed by others
References
Ohshima H, Furusawa K (eds) (1998) In: Electrical phenomena at interfaces. 2nd edn. Marcel Dekker, New York
Masliyah JH, Bhattacharjee S (2006) Electrokinetic and colloid transport phenomena, 1st edn. Wiley, Hoboken
Elimelech M, Gregory J, Jia X, Williams RA (1998) Particle deposition & aggregation, paperback edn. Butterworth-Heinemann, Woburn
von Smoluchowski M (1903) Bull Int Acad Sci Cracov 8:182–200
Henry DC (1931) Proc R Soc Lond 133A:106–129
Huckel E (1924) Phys Z 25:204–210
Booth F (1950) Proc R Soc Lond 203A:514–533
Overbeek JThG (1943) Kolloide Beihefte 54:287–364
Wiersmema PH, Loeb AL, Overbeek JThG (1966) J Colloid Interface Sci 22:78–99
O’Brien RW, White LR (1978) J Chem Soc Faraday Trans 2(74):1607–1626
Ohshima H, Healy TW, White LR (1983) J Chem Soc Faraday Trans 2(79):1613–1628
Ohshima H (2005) Colloids Surf A 267:50–55
O’Brien RW, Hunter RJ (1981) Can J Chem 59:1878–1887
Dukhin SS, Semenikhin NM (1970) Kolloid Zh 32:360–368
Hidalgo-Alvarez R, Martin A, Fernandez A, Bastos D, Martinez F, de las Nieves (1996) Adv Colloid Interface Sci 67:1–118
Elimelech M, O’Melia CR (1990) Colloids Surf 44:165–178
Bastos-Gonzalez D, Hidalgo-Alvarez R, de las Nieves FJ (1996) J Colloid Interface Sci 177:372–379
Borkovec M, Behrens SH, Semmler M (2000) Langmuir 16:2566–2575
Antonietti M, Vorwerg L (1997) Colloid Polym Sci 275:883–887
Bastos D, de las Nieves FJ (1993) Colloid Polym Sci 271:860–867
Quesada-Perez M, Gonzarez-Tovar E, Martin-Molina A, Lozada-Cassou M, Hidalgo-Alvarez R (2005) Colloids Surf A 267:24–30
Martin-Molina A, Quesada-Perez M, Galisteo-Gonzalez F, Hidalgo-Alvarez R (2004) Prog Colloid Polym Sci 123:114–118
Labbez C, Nonat A, Isabelle P, Jonsson B (2007) J Colloid Interface Sci 309:303–307
Behrens SH, Christl DI, Emmerzael R, Schurtenberger P, Borkovec M (2000) Langmuir 16:5209–5212
Lin W, Kobayashi M, Skarba M, Mu C, Galletto P, Borkovec M (2006) Langmuir 22:1038–1047
Chow RS, Takamura K (1988) J Colloid Interface Sci 125:212–225
Chow RS, Takamura K (1988) J Colloid Interface Sci 125:226–236
Malvern Instrument (2004) Zetasizer Nano series user manual
Ohshima H (2006) Theory of colloid and interfacial electronic phenomena, 1st edn. Academic, London
Morisaki H, Nagai S, Ohshima H, Ikemoto E, Kogure K (1999) Microbiology 145:2797–2802
Lide DR (ed) (2001) In: CRC handbook of chemistry and physics. 82th edn. CRC, Boca Raton
The Chemical Society of Japan (ed) (2004) In: Kagaku Binran. 5th edn. Maruzen, Tokyo
Israelachvili JN (1992) Intermolecular and surface forces, 2nd edn. Academic, London
Acknowledgement
This work was financially supported by the MEXT KAKENHI (18688013).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kobayashi, M. Electrophoretic mobility of latex spheres in the presence of divalent ions: experiments and modeling. Colloid Polym Sci 286, 935–940 (2008). https://doi.org/10.1007/s00396-008-1851-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-008-1851-9