Abstract
Transient receptor potential melastatin 4 (TRPM4) cation channels act in cardiomyocytes as a negative modulator of the L-type Ca2+ current. Ubiquitous Trpm4 deletion in mice leads to an increased β-adrenergic inotropy in healthy mice as well as after myocardial infarction. In this study, we set out to investigate cardiac inotropy in mice with cardiomyocyte-specific Trpm4 deletion. The results guided us to investigate the relevance of TRPM4 for catecholamine-evoked Ca2+ signaling in cardiomyocytes and inotropy in vivo in TRPM4-deficient mouse models of different genetic background. Cardiac hemodynamics were investigated using pressure–volume analysis. Surprisingly, an increased β-adrenergic inotropy was observed in global TRPM4-deficient mice on a 129SvJ genetic background, but the inotropic response was unaltered in mice with global and cardiomyocyte-specific TRPM4 deletion on the C57Bl/6N background. We found that the expression of TRPM4 proteins is about 78 ± 10% higher in wild-type mice on the 129SvJ versus C57Bl/6N background. In accordance with contractility measurements, our analysis of the intracellular Ca2+ transients revealed an increase in ISO-evoked Ca2+ rise in Trpm4-deficient cardiomyocytes of the 129SvJ strain, but not of the C57Bl/6N strain. No significant differences were observed between the two mouse strains in the expression of other regulators of cardiomyocyte Ca2+ homeostasis. We conclude that the relevance of TRPM4 for cardiac contractility depends on homeostatic TRPM4 expression levels or the genetic endowment in different mouse strains as well as on the health/disease status. Therefore, the concept of inhibiting TRPM4 channels to improve cardiac contractility needs to be carefully explored in specific strains and species and prospectively in different genetically diverse populations of patients.
Similar content being viewed by others
Introduction
During cardiac contraction cardiomyocytes of the working myocardium undergo electro-mechanical coupling. With extracellular electrical excitation, voltage-gated L-Type Ca2+ channels in the membrane of the transverse tubules opens followed by a Ca2+ influx, which elevates [Ca2+]i and thereby activates calcium-induced calcium release (CICR) initiating myocardial contraction [4, 8]. Cardiac contractility is influenced by numerous regulatory mechanisms, including the adjustment by the sympathetic nervous system. β-adrenergic stimulation by catecholamines activates the adenylyl cyclase (AC) which converts ATP to cAMP. An elevation in cytosolic cAMP level in cardiomyocytes leads to the activation of cAMP-dependent protein kinase A (PKA), which increases the activity of various effector proteins [20, 32]. PKA phosphorylation of the voltage-gated L-type Ca2+ channel, the ryanodine receptor RyR2 and Phospholamban, which relieves the inhibition of the sarcoplasmic reticulum (SR) Ca2+ ATPase SERCA2a, increases the intracellular Ca2+ influx during systole and provide more rapid reuptake of Ca2+ ions into the sarcoplasmic reticulum during diastole [4, 15]. Consequently, in the following systole, a larger Ca2+ transient is generated resulting in a higher contraction force [29].
Expression of the calcium-activated monovalent cation channel Transient Receptor Potential Melastatin 4 (TRPM4) has been found in the heart of humans as well as in the atrium and ventricle of the mouse heart. It is expressed in cardiomyocytes, cardiac conduction system as well as in endothelial cells and smooth muscle cells [5, 11, 16, 17, 22, 26]. The gain of function mutations of the Trpm4 gene are strongly associated with familial arrhythmia syndromes such as Brugada syndrome and the progressive familial heart block type I [16, 18, 34]. Insights from Trpm4-deficient cardiomyocytes have identified TRPM4 as a negative modulator of the L-type Ca2+ influx under β-adrenergic stimulation, and its deletion leads to an increased action potential duration and thereby results in an enhanced driving force for Ca2+ through the L-Type calcium channel. Consequently, systolic Ca2+ transients are elevated under β-adrenergic stimulation, and contractility of isolated left ventricular papillary muscles showed that β-adrenergic stimulation leads to an increased contractile force in TRPM4-deficient mice compared to wildtype controls [21, 37]. Furthermore, hemodynamic measurements under increasing β-adrenergic stimulation with isoproterenol resulted in a stronger left ventricular inotropic response in TRPM4-deficient mice compared to controls. However, under basal conditions, no significant differences were reported [21]. In addition, an enhanced β-adrenergic cardiac reserve was observed in TRPM4-deficient mice under conditions of ischemia evoked heart failure compared to wildtype controls [10]. These studies suggest a pharmacological or genetic inactivation of TRPM4 as a potential treatment strategy to enhance cardiac output in patients with severe heart failure with elevated catecholamine levels.
Methods
Mice and genotyping
Experimental procedures were approved by the regional council Karlsruhe according to the Animal Welfare Act (T-64/18, G-89/15), and by the Animal Ethics Committee of the KU Leuven (In Vitro-Vennekens). TRPM4-deficient mouse lines were described previously [39]. Breeding and housing of TRPM4-deficient mice and littermate controls were performed in the Interfaculty Biomedical Faculty (IBF) of the University of Heidelberg. Mice were kept under specified pathogen-free conditions on a 12-h light/12-h dark cycle with water and standard food (Rod18, LASvendi GmbH, Germany) available ad libitum. Experiments were performed in 18–19 weeks old male mice. Trpm4+/– mice [39] were independently backcrossed to the 129SvJ and C57Bl/6N background for 6 and 10 generations respectively, before Trpm4+/– mice on either background were bred to obtain Trpm4–/– mice and corresponding Trpm4+/+ litter matched controls (termed Trpm4–/–(Bl/6N) and Trpm4+/+(Bl/6N) throughout the manuscript). Trpm4–/–(129SvJ) and Trpm4+/+(129SvJ) littermatched control mice on the 129SvJ background were obtained by analogous breeding. The generation of Trpm4+/flox mice was also reported previously [39]. After 9 generations of backcrossing to the C57Bl/6N strain, Trpm4+/flox mice were mated with αMHC-CreERT2 mice [36] (also on the C57Bl/6N background). To induce Cre recombinase-mediated deletion of exons 15 and 16 of the Trpm4 gene selectively in cardiomyocytes of adult mice, a daily tamoxifen injection was performed on five consecutive days on 6 weeks old Trpm4flox/flox; αMHC-CreERT2 positive (Crepos) mice (termed Trpm4iCM−KO). Trpm4flox/flox; αMHC-CreERT2 negative (Creneg) littermates were treated with tamoxifen as well and served as controls.
The identification of the genotype of the experimental mice was carried out by PCR using the primer pairs listed in Table 1. Ear biopsies were transferred into 97.5 μl of DirectPCR buffer (Viagen, USA) with 2.5 μl proteinase K (10 mg/ml, AppliChem, Germany) and lysed overnight at 55 °C. Subsequently, the proteinase K was denatured for 45 min at 85 °C.
Microsomal membrane preparation
Mice were sacrificed using cervical dislocation. The thorax was opened and the still-beating heart was washed out with NaCl 0.9% (Braun, Germany) injected via the apex. Mouse hearts were transferred in PBS and atria were removed. Ventricular tissue was homogenized in 1 ml lysis buffer (100 mM TRIS–HCl; 1 mM MgCl2; pH 8.0 supplemented with 1 mM Iodacetamide; 1 mM Phenantholin; 0.1 mM PMSF; 1 µg/ml Antipain; 1 µg/ml Leupeptin; 0.7 µg/ml Pepstatin; 1 mM Benzamidine; 0.3 µM Aprotinin, Applichem, Germany) using a 2 ml tissue grinder (Tenbroeck Tissue Grinders, Wheaton). Homogenates were filled up to 3 ml with lysis buffer and were frozen at − 80 °C for 20 min following by thawing on ice for about 1 h. Afterwards 15 ml of sucrose buffer (0.25 M sucrose; 10 mM TRIS–HCl; pH 7.4 supplemented with 1 mM Iodacetamide; 1 mM Phenantholin; 0.1 mM PMSF; 1 µg/ml Antipain; 1 µg/ml Leupeptin; 0.7 µg/ml Pepstatin; 1 mM Benzamidine; 0.3 µM Aprotinin, Applichem, Germany) was added and the samples were centrifuged for 30 min at 6000×g. Subsequently, supernatant was centrifuged at 50.000×g for 1 h 45 min. The resulting microsomal membrane pellet was solved in 200 µl sucrose buffer. Protein concentration was determined by BCA assay (Thermofisher Scientific USA; 23227). Microsomal membrane fractions were supplemented with 4 × Laemmli buffer (60 mM TRIS–HCl; 10% (v/v) Glycerol; 5% (v/v) β-mercaptoethanol; 4% (w/v) SDS; 0.005% (w/v) Bromophenol blue; pH 6.8) and incubated at 60 °C for 20 min. Afterwards microsomal membrane fractions were stored at − 80 °C.
Immunoblotting
Microsomal membrane fractions (50 µg/well) were loaded on a 10% Bis–Tris Plus gel (Invitrogen, USA; NW04122) followed by gel electrophoresis at 130 V for 90 min. Resolved proteins were electroblotted onto a 0.45 µm Protran nitrocellulose membrane (GE Healthcare, USA; A10195922) at 12 mV for 60 min. Afterwards the membrane was blocked in 5% non-fat milk with TBST (50 mM TRIS–HCl; 150 mM NaCl; 0.1% (v/v) Tween-20; pH 7.5). Blots were incubated in anti-GAPDH 1:500 (Acris, USA; ACR001P), anti-TRPM4 [39] 1:100 (ab578 provided by Veit Flockerzi), anti-Cavβ2 [23] 1:100 (ab425 provided by Veit Flockerzi) or anti-SERCA2a (Badrilla, UK; A010-23L) at 4 °C for 24 h. Blots were washed three times for 10 min in TBST and subsequently incubated in horseradish peroxidase-conjugated anti-rabbit 1:50.000 (GE Healthcare, USA; NA9340V) at RT for 2 h. Blots were washed two times for 10 min in TBST and once for 10 min in TBS (50 mM TRIS–HCl; 150 mM NaCl; pH 7.5). Blots were incubated in SignalFire Elite ECL (Cell Signaling, USA) for 1 min and chemoluminescence was detected by digital imaging (GE Healthcare, ImageQuant LAS 4000 mini). Protein expression levels were determined by densitometry analysis using ImageJ software.
Cardiac hemodynamic measurements
Mouse ventricular function was measured by a miniaturized pressure-conductance catheter. Mice were anesthetized by peritoneal injection of an anesthetic-analgesic mixture of 1200 mg/kg Urethane (Sigma-Aldrich, USA), 50 mg/kg α-Chloralose (Sigma-Aldrich, USA), and 0.1 mg/kg Buprenorphine (Bayer AG, Germany). Anesthetized mice were intubated and mechanically ventilated with 100% O2 by a small rodent respirator (MiniVent 845, Harvard Apparatus, USA). Body temperature was monitored using a rectal temperature probe (Hugo Sachs Electronic, Germany) and maintained at 37 ± 0.2 °C by placing the mouse on a heating plate (Hotplate 062, Labotect). Upon the absence of interdigital reflexes, 1 μg/g of the muscle relaxant Pancuronium (Sigma-Aldrich, USA) was administered intraperitoneally. A central venous catheter was placed in the left femoral vein for continuous infusion (15 μl/min; 11Plus, Harvard Apparatus, USA) of NaCl 0.9% (Braun, Germany) and for the application of different isoproterenol concentrations for β-adrenergic stimulation during the measurement [1]. After performing a limited thoracotomy with an electric cautery pen (Faromed, Germany), the Pressure–Volume loop (PV loop) catheter SPR-839 (Millar, USA) was placed into the mouse left ventricular cavity via the apex. Optimal placement of the catheter was followed by a stabilization phase of 10 min. Preload dependent parameters were recorded by an expiratory ventilation break of max. 5 s. To assess preload independent contractility, the inferior caval vein was occluded and five consecutive PV loops were recorded during preload reduction to analyze Preload-Recruitable Stroke Work (PRSW). After basal recordings followed the β-adrenergic stimulation by infusion of increasing concentrations of the positive inotropic substance isoproterenol (9, 30, (60), 90 to a maximum of 300 ng/kg/min) [21, 27]. At the end of each measurement, the parallel conductance of the myocardium was determined by an intravenous bolus injection of 15 μl of a 15% hypertonic NaCl solution. Cuvette calibration of heparinized blood from each mouse was used for conductance (µS) to blood volume (µl) conversion.
Isolation of ventricular cardiac myocytes
10- to 12 weeks-old mice were heparinized and sacrificed by intraperitoneal injection of sodium pentobarbital (Dolethal, Vétoquinol, UK). Hearts were quickly removed and perfused retrograde through the aorta with O2-saturated Ca2+-free Tyrode solution containing (in mM): 117 NaCl, 4 KCl, 1 KH2PO4, 4 NaHCO3, 10 HEPES, 10 Glucose and 1.7 MgCl2 (pH = 7.4 with NaOH). Afterwards, the heart was washed with Tyrode solution containing 0.23 mM EGTA. This step was followed by 5–10 min perfusion of Tyrode solution containing 100 µM Ca2+ and Collagenase B (Worthington). Next, the heart was removed from the Langendorff apparatus, atria were discarded and ventricular muscle was cut into small pieces in fresh enzyme solution supplemented with 300 µM Ca2+. This suspension was gently agitated for 2–3 min, filtered through a 100 µM nylon mesh and centrifuged at 400 rpm for 3 min. The resulting pellet was washed with Tyrode solution containing 0.5 mM Ca2+ and BSA. The suspension was left for decantation for 3–7 min and the pellet was washed again with Tyrode solution containing 1 mM Ca2+ and BSA. Myocytes were kept at room temperature and used for experiments for 7 h.
Calcium imaging
Cardiomyocytes were loaded with 5 µM Fluo-4 AM for 30 min at 37 °C. After placing Fluo4- loaded cells in a recording chamber, cells were constantly perfused with an extracellular solution containing (in mM): 95 NaCl, 4.8 KCl, 24.88 NaHCO3, 1.18 KH2PO4, 10 Glucose, 1.5 CaCl2 and 1.87 MgCl2. This solution was continuously bubbled with carbogen (5% CO2, 95% O2). Measurements were performed at a temperature of 32 °C using a single channel bipolar temperature controller (CL-100) (Warner Instruments, USA). Electrical stimulation was achieved via local field stimulation using a custom-made electrode. Stimulation was provided by 2 ms pulses of 3 V at 5 Hz using a MyoPacer EP Field stimulator (IonOptix, Netherlands). Fluorescence was detected using a photo-multiplier tube and was acquired by the Patchmaster (v2 × 75) software (HEKA Elektronik, Germany). Excitation light (477 nm) was provided by a Polychrome V monochromator (TILL Photonics, Germany). Data were sampled at 5 kHz. Fluorescence is shown as: F = (F–F_1)/F_0, with F0 = basal Ca2+-signal, and F1 = Ca2+-signal 2 ms before the occurrence of each Ca2+-transient. In the analysis, F2 represented the peak fluorescence of the Ca2+-transient. The results are displayed as averaged values from cells of biologically independent mice (10 WT and 9 KO animals on C57Bl/6N and 8 WT and 10 KO animals on 129SvJ background, respectively).
Statistics
Statistical analyses were performed using OriginPro 8.5 and Excel 2010. Data are expressed as mean ± standard error of the mean (SEM) or mean ± standard deviation (SD). Normality distribution of the data was tested using a Shapiro–Wilk test and considered normally distributed if p > 0.05. The difference between the two groups was tested using a Two-Sample T-test or its non-parametric alternative, Mann–Whitney test. P-values < 0.05 were considered statistically significant.
Results
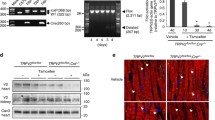
Previous data from our group indicate that TRPM4 global knockout mice have an improved cardiac inotropic response to β-adrenergic stimulation [21]. To test whether the deletion of the Trpm4 gene specifically in cardiomyocytes improves cardiac contractility in healthy mice, we generated mice with a αMHC-Cre dependent deletion of the Trpm4 gene. To analyze the efficiency of cardiomyocyte-specific Trpm4 deletion in Trpm4flox/flox; αMHC-Crepos (Trpm4iCM−KO) mice, we isolated microsomal membrane fractions from hearts of three independent Trpm4flox/flox; αMHC-Crepos and Trpm4flox/flox; αMHC-Creneg mice, respectively, and performed Western Blot analysis. Proteins of ∼ 136 kDa were detected in the heart of Trpm4flox/flox; αMHC-Creneg using ab478, which identified TRPM4 specifically in other cells and tissues from WT but not from the corresponding Trpm4–/– controls [22]. The size of these proteins is within the expected size range for TRPM4 proteins and the expression level of these proteins was reduced by 78 ± 10% in Trpm4iCM−KO mice. Trpm4–/– mice entirely lacking TRPM4 proteins served as control (Fig. 1a). Using these mice, cardiac function was assessed using Pressure–Volume loop (PV-loop) measurements. After recordings of basal heart function, acute β-adrenergic stimulation with isoproterenol in Trpm4iCM−KO and Creneg control mice was performed (Fig. 2a and b). A gradual increase in isoproterenol concentrations from 9 to 300 ng/kg/min resulted in the expected rise in heart rate (Fig. 2c) and systolic parameters such as stroke volume (Fig. 2d) stroke work (Fig. 2e) and the contractility parameter Pre-recruitable Stroke Work (PRSW) (Fig. 2f). Additional parameters describing cardiac function were analyzed and are listed in Table 2. However, the results of the functional measurements showed no significant difference in cardiac contractile function between Crepos and Creneg animals.
Protein expression analysis of TRPM4 in adult Trpm4flox/flox; αMHC-CreERT2 mice and constitutive Trpm4-deficient mice on the genetic C57Bl/6N or 129SvJ background. a Western blots (left) with anti-TRPM4 and anti-GAPDH, 10 weeks after tamoxifen treatment; right panel: Analysis of the relative expression analysis shows a TRPM4 reduction of 78 ± 10% in the heart of Trpm4flox/flox; αMHC-CreERT2 pos. animals. Microsomal membrane fractions from n = 4 independent Cre negative and Cre positive mice were analyzed. b Western blot analysis with anti-TRPM4, anti-SERCA2a, anti-Cavβ2 and anti-GAPDH in globally Trpm4–/–(Bl/6N) (n = 4) and Trpm4–/–(129SvJ) (n = 3) mice compared to Trpm4+/+(Bl/6N) (n = 4) and Trpm4+/+(SvJ) (n = 3) littermate controls. Relative expression of TRPM4 (c), Cavβ2 (d) and SERCA2a (e). Trpm4+/+(Bl/6N) Values are expressed as means ± SD. *p < 0.05, **p < 0.01
Measurement of cardiac function of inducible and cardiomyocyte-specific TRPM4-deficient mice on the C57Bl/6N genetic background. Representative pressure–Volume loops under basal conditions and β-adrenergic stimulation of a Trpm4flox/flox/αMHC-CreERT2 negative (n = 4) and b Trpm4flox/flox/αMHC-CreERT2 positive (n = 6) littermates. Statistical analysis of isoproterenol dose–response curves of c heart rate, d stroke volume, e stroke work and f PRSW (Preload-Recruitable-Stroke Work). Values are expressed as means ± SEM
Since we did not find a difference in mice lacking Trpm4 selectively in cardiomyocytes we analyzed Trpm4 global knockout mice on the same C57Bl/6N genetic background (Trpm4–/–(Bl/6N) and litter matched Trpm4+/+(Bl/6N) as controls) as we did with the Trpm4iCM−KO before. Western Blot analysis of microsomal membrane fractions of Trpm4+/+(Bl/6N) again showed a single band (∼ 136 kDa) whereas these proteins were not detected in the corresponding Trpm4–/–(Bl/6N) hearts (Fig. 1a, c). After measuring the basal cardiac function, acute β-adrenergic stimulation via the venous catheter followed. A gradual increase in isoproterenol concentrations from 9 to 300 ng/kg/min led to the expected β-adrenergic dose–response relationship in TRPM4−/−(Bl/6N) mice as well as in TRPM4+/+(Bl/6N) littermates (Fig. 3a and f). Again, the analysis of the functional measurements showed no significant differences in heart rate (Fig. 3c) nor in the systolic parameters stroke volume (Fig. 3d), stroke work (Fig. 3e) or the contractility parameter PRSW (Fig. 3f) between TRPM4−/−(Bl/6N) and TRPM4+/+(Bl/6N) mice. Additional parameters describing cardiac function were analyzed and are listed in Table 3.
Measurement of cardiac function in global TRPM4 knockout mice on the C57Bl/6N genetic background. Representative pressure–Volume loops under basal conditions and β-adrenergic stimulation of a Trpm4+/+(Bl/6N) mice (n = 8) and b Trpm4–/– (Bl/6N) (n = 7) littermates. Statistical analysis of isoproterenol dose–response curves of c heart rate, d stroke volume, e stroke work and f PRSW (Preload-Recruitable-Stroke Work). Values are expressed as means ± SEM
Since all cardiac functional parameters analyzed were unaltered in Trpm4–/–(Bl/6N) mice compared to corresponding Trpm4+/+(Bl/6N) controls, we wanted to determine whether the differences to previously published results showing increased contractility in Trpm4–/– mice on a 129SvJ background upon isoproterenol treatment in PV loop measurements [21] and isolated papillary muscle preparations [37], were due to the different genetic background. Thus, we evaluated Trpm4–/–(129SvJ) mice in comparison to litter matched Trpm4+/+(129SvJ) controls. Western Blot analysis in this mouse line showed an abundant expression of ∼ 136 kDa proteins in Trpm4+/+(129SvJ) mice, whereas these proteins were absent in Trpm4–/–(129SvJ) mice (Fig. 1b, c). Again, measurements of basal heart function were followed by acute β-adrenergic stimulation via infusion of increasing isoproterenol concentrations from 9 to 300 ng/kg/min, which resulted in expected dose-dependent changes in PV loops in TRPM4−/−(129SvJ) mice as well as in TRPM4+/+(129SvJ) littermates (Fig. 4a and f). The evaluation of the functional parameters showed no change in heart rate between TRPM4−/−(129SvJ) and TRPM4+/+(129SvJ) littermates (Fig. 4c). In contrast, a significantly increased β-adrenergic inotropic response in stroke volume (Fig. 4d) and in the left ventricular contractility parameter PRSW (Fig. 4f), which represents a key parameter to characterize cardiac function independent of preload and afterload [1, 9], was found in TRPM4−/−(129SvJ) mice compared to TRPM4+/+(129SvJ) control mice. Additional parameters describing cardiac function were analyzed and are listed in Table 4.
Measurement of cardiac function in global TRPM4 knockout mice on the 129SvJ genetic background. Representative pressure–Volume loops under basal conditions and β-adrenergic stimulation of a Trpm4+/+(129SvJ) mice (n = 7) and b Trpm4–/–(129SvJ) (n = 6) littermates. Statistical analysis of isoproterenol dose–response curves of c heart rate, d stroke volume, e stroke work and f PRSW (Preload-Recruitable-Stroke Work). Values are expressed as means ± SEM. *p < 0.05, **p < 0.01
To evaluate whether the differences in inotropic response in Trpm4–/–(129SvJ) and Trpm4/–(Bl/6N) mice after β-adrenergic receptor stimulation in comparison to their corresponding Trpm4+/+ controls are due to changes in Ca2+ signaling in cardiomyocytes isolated from the different mouse strains, we performed measurements of [Ca2+]i in electrically paced cardiomyocytes from Trpm4–/–(Bl/6N) and Trpm4–/–(129SvJ) mice and WT littermates (Fig. 5a, b, f and g). The resting Ca2+ values (F1/F0) were identical between TRPM4 knockout and wildtype animals both in the 129SvJ and C57Bl/6N strain (resting Ca2+ values in C57Bl/6N strain: Trpm4+/+(Bl/6N): 1.25 ± 0.11 (n = 10), Trpm4–/–(Bl/6N) 1.33 ± 0.4 (n = 9), resting Ca2+ values in C57Bl/6N strain before iso stimulation: Trpm4+/+(Bl/6N) 1.22 ± 0.13 (n = 10), Trpm4–/–(Bl/6N) 1.33 ± 0.1 (n = 9), resting Ca2+ values in 129SvJ strain: Trpm4+/+(129SvJ) 1.34 ± 0.1 (n = 8), Trpm4–/–(129SvJ) 1.35 ± 0.17 (n = 10), resting Ca2+ values in 129SvJ strain before iso stimulation: Trpm4+/+(129SvJ) 1.45 ± 0.1 (n = 8), Trpm4–/–(129SvJ) 1.45 ± 0.17 (n = 10). During steady-state electrical pacing the amplitude of electrically evoked Ca2+ transients (systolic Ca2+ rise) was not different between Trpm4–/–(Bl/6N) and Trpm4+/+(Bl/6N) cardiomyocytes (Fig. 5c). Furthermore, after catecholamine stimulation with isoproterenol (1 µM) the amplitude of electrically evoked Ca2+ transients was significantly increased to the same extent in Trpm4–/–(Bl/6N) and Trpm4+/+(Bl6//N) cardiomyocytes (Fig. 5d). In Trpm4–/–(129SvJ) cardiomyocytes the amplitude of electrically evoked Ca2+ transients were not different compared to Trpm4+/+(129SvJ) cardiomyocytes under steady-state electrical pacing. However, the effect of catecholamine stimulation with isoproterenol (1 µM) (Fig. 5h and i) was enhanced in Trpm4–/–(129SvJ) versus Trpm4+/+(129SvJ) cardiomyocytes, which was in line with previously reported findings in Trpm4–/– mice of the 129SvJ genetic background [21].
Analysis of Ca2+ transient in cardiomyocytes from Trpm4+/+ and Trpm4−/− mice on the C57Bl/6N (a–e) or 129SvJ (f–j) background. (a–b) representative examples illustrating the time-course of experiments to test the effect of isoprenaline (1 µM) on electrically evoked Ca2+ transients (5 Hz). After the application of isoprenaline, the pacing was seized and cells were stimulated with caffeine (10 mM) to induce Ca2+ release. Analysis of the basal amplitude of electrically-evoked Ca2+ transients (c), the relative effect of isoprenaline (d) and the amplitude of the caffeine evoked transient (e) in cells from Trpm4+/+ and Trpm4−/− cardiomyocytes from C57Bl/6 N mice. f–j show similar experiments but from cardiomyocytes from 129SvJ mice. Amplitudes of Ca2+ transients were calculated as follows: F2 represents the peak of the Ca2+ transient, F1 is the base of the Ca2+ transient, and F0 is the fluorescence value in a resting cell, before the cell is stimulated. Thus (F2-F1)/F0 gives the net amplitude of the Ca2+ transients normalized to the loading of the cell (F0). In all our traces background fluorescence (fluorescence value of a cell-free field) is subtracted from the data. Each data point represents averaged values from cells of biologically independent mice (10 WT and 9 KO animals on C57Bl/6N and 8 WT and 10 KO animals on 129SvJ background. Values are expressed as means ± SEM. **p < 0.01
Since differences in Ca2+ signaling and contractility have been reported between C57Bl/6N and 129SvJ mouse strains before, which were attributed to differences in the expression of regulators of cardiomyocyte Ca2+ homeostasis such as voltage-gated L-type channels, the SERCA2a ATPase or others [41] we tested whether the expression of SERCA2a and of Cavβ2, the predominant accessory subunit of cardiac L-type channels [23, 42], might be differently expressed between cardiomyocytes from the two mouse strains. However, the densitometric analysis revealed no significant differences in these proteins between the two mouse strains (Fig. 1d, e). Notably, we observed significant differences in the abundance of TRPM4 proteins, which were 78 ± 10% higher in wild-type mice on the 129SvJ genetic background compared to the C57Bl/6N strain (Fig. 1c). Furthermore, we found no difference in the caffeine-evoked Ca2+ amplitude between cardiomyocytes of the 129SvJ strain and the C57Bl/6N strain indicating that the amount of Ca2+ which can be released from the SR is not different in cardiomyocytes of these two mouse strains (Fig. 5e, j).
Discussion
Our previous studies have suggested that the activation of the cation channel TRPM4 in cardiomyocytes reduces the driving force for L-type mediated Ca2+ influx. The global deletion of Trpm4 in the mouse model resulted in increased cardiac contractility under β-adrenergic stimulation, both under healthy conditions [21, 37] and after induction of myocardial infarction [12]. Our current results suggest that in healthy mice this effect is critically dependent on the genetic background of the mouse model being studied. Indeed, both in an inducible cardiomyocyte-specific Trpm4flox/flox; αMHC-CreERT2 mouse model as well as in globally TRPM4-deficient mice on the same C57Bl/6N genetic background no increase in inotropy during β-adrenergic stimulation could be detected. Of note, a difference in the protocol used in this study is that the pressure–volume catheter is placed in the left ventricle via the apex, while in Mathar et al. the catheter was introduced into the left ventricle via the carotid artery with the chest closed [21]. The latter, previously published, PV loop measurements showed an increased ISO-evoked improvement of contractility in Trpm4–/– mice on the 129SvJ background [21], which we could faithfully reproduce in this study, in global Trpm4–/–(129SvJ) mice in comparison to Trpm4+/+(129SvJ) littermates using open-chest insertion of the catheter via the apex. Determination of left ventricular function revealed a significantly increased β-adrenergic inotropy, as well as a significant increase in stroke volume during isoproterenol stimulation in Trpm4–/–(129SvJ) mice in comparison to Trpm4+/+(129SvJ), controls. This indicates that the difference in the methodological approach cannot explain why an increase in ISO-evoked cardiac contractility was not observed upon TRPM4 deletion in healthy mice of the C57Bl/6N strain in our study. Since this phenomenon was confined to Trpm4–/–(129SvJ) mice, we conclude that the phenotype of increased β-adrenergic inotropy depends on the genetic background, as well as on the health/disease status of the mice.
The influence of genetic background on cardiac functions has been demonstrated in several other studies. The genetic homogeneity of inbred strains provides ideal conditions for the investigation of causal physiological relationships. The most commonly used genetic background in the analysis of cardiac function is that of the C57Bl/6 mouse, the first mouse strain that was fully sequenced [24]. Most genetically modified mouse lines were generated from embryonic stem cells from inbred strains of the 129 background and then backcrossed to the genetic background of C57Bl/6 mouse strains. However, there are genetic divergences between different inbred strains, which are often paid little attention and can lead to confusing results when comparing different inbred strains [6]. For example, C57Bl/6 mouse strains have a single renin gene, Ren-1c, while 129SvJ mice have two renin genes, Ren-1d and Ren-2. This is associated with e.g. higher blood pressure in 129SvJ compared to C57Bl/6 mice [19]. Further studies reported differences in cardiac function between different inbred strains [2, 3, 27, 30, 31, 35, 38, 41]. Hemodynamic analysis of eight commonly used inbred mouse strains showed differences in cardiac contractility that were pronounced during acute hypoxia or β-adrenergic blockade [2]. The β-adrenergic blockade inhibited cardiac function more strongly in 129X1/SvJ than in the C57Bl/6J strain suggesting that 129X1/SvJ mice have a higher sympathetic basal tone than C57Bl/6J mice, which in addition would explain desensitization-induced lower cardiac β1 adrenoceptor density in 129X1/SvJ animals compared to C57Bl/6J mice [31]. Differences in contractility and relaxation between the 129SvJ and C57Bl/6 J strain with and without ISO treatment were reported using hemodynamic measurements [41]. Similar studies with comparative analysis of the cardiac contractile function of the C57Bl/6N strain have not been reported to our knowledge. The distinction between N and J strain is of importance for the analysis of cardiac function particularly in the disease state. A recent study could demonstrate in detail that the defective mitochondrial transhydrogenase (NnT) in C57Bl/6J ameliorates pressure overload-induced cardiac failure because of differences in antioxidative capacity [25].
Interestingly, differences in the expression of proteins affecting the intracellular Ca2+ transient were also observed between different inbred strains. Quantitative PCR analysis showed a significantly higher expression of RyR2 and an enhanced expression of SERCA2a in 129/SvJ mice compared to C57Bl/6 [41]. This could potentially affect intracellular Ca2+ homeostasis, as Shah and colleagues had pointed out. They recognized that cardiomyocytes from 129/SvJ mice have a higher sarcoplasmic reticulum (SR) “Ca2+-load” and a lower SR “Ca2+-leak” compared to C57Bl/6 mice [31]. However, under a frequency-dependent stimulation, no increase in systolic Ca2+ amplitude could be detected between the cardiomyocytes of the two strains, as well as no difference in SR Ca2+ release [31]. Also, in our hands, no obvious differences in the Ca2+ handling between 129SvJ and C57Bl/6N cardiomyocytes could be observed. In contrast, we found that the expression of TRPM4 proteins is about 78 ± 10% higher in wild-type mice on the 129SvJ genetic background compared to the C57Bl/6N strain. This interesting finding could indicate that the TRPM4 protein is more physiologically relevant, particularly with respect to inotropic response, in the left ventricle of 129SvJ mice than in C57Bl/6N mice. Mathar et al. suggested that the inotropic phenotype of TRPM4−/− mice depends essentially on a modification of Ca2+ dependent excitation–contraction coupling in TRPM4−/− mice. They found that the ISO-induced increase of the evoked Ca2+ transient is significantly larger in TRPM4−/− cardiomyocytes, compared to WT cardiomyocytes. Considering that differences in Ca2+ signaling in cardiomyocytes from different mouse strains have been shown before [31], we studied catecholamine evoked rise in systolic Ca2+ transients in cardiomyocytes of both genotypes and mouse strains. Whereas the ISO-evoked increase of the Ca2+ transient was significantly larger in TRPM4−/− cardiomyocytes in the 129SvJ strain compared to TRPM4+/+ controls, which reinforces our previous analysis [21], we could not find a significant difference between the two genotypes in the C57Bl/6 N background. In our attempts to gain mechanistic insights into the differences in TRPM4-dependent Ca2+ signaling between the two strains, we analysed protein expression of the L-Type Ca2+ channel subunit CaVβ2 and SERCA2a proteins, whose expression level and activity regulate diastolic SR Ca2+ load [7]. However, our findings show neither divergences in CaVβ2 and SERCA2a protein expression nor differences in caffeine-induced SR Ca2+ depletion in cardiomyocytes from 129SvJ compared to C57Bl/6N mice indicating that SR Ca2+ load is not different in ventricular cardiomyocytes between the strains. Taken together, these results confirm our previous analysis. The finding that cardiac TRPM4 expression in C57Bl/6N was drastically (∼ 80%) lower compared 129SvJ mice suggests that TRPM4 is a relatively less important determinant of the driving force of L-type Ca2+ entry in C57Bl/6N cardiomyocytes, as compared to 129SvJ cardiomyocytes.
However, we cannot exclude that also other factors that determine ISO-evoked Ca2+ rise and cardiac contractility on the organismal level might be different between TRPM4-deficient mice on the respective genetic background. An example of such discrepancies was reported in the study of Vignier et al. who investigated the LMNA p.H222P mutation leading to Emery-Dreifuss muscular dystrophy (EDMD) in humans in C57BL/6JRj and 129S2/SvPas background. This mutation is associated with arrhythmia-related symptoms resulting in cardiac dilation and heart failure. In contrast to C57Lmna p.H222P mice, 129Lmna p.H222P mice showed exacerbated arrhythmia susceptibility making 129Lmna p.H222P mice a more relevant model for evaluating therapeutic strategies for EDMD [40]. The studies above as well as our findings emphasize that the analysis of null alleles or disease-related mutations in multiple mouse strains is recommendable. Ultimately, the most adequate species and strain need to be chosen to study distinct physiological or pathophysiological processes [2, 13, 38, 41] and to test potential therapeutic strategies. Obviously, the 129SvJ strain is more suitable to study TRPM4-dependent processes in the heart compared to the C57Bl/6N strain where the homeostatic expression level is comparably low.
Overall, it can be concluded that a genotype–phenotype context of an isolated genetic background is not easily generalized for different inbred mouse strains [28, 33]. F1 hybrids could be a useful alternative to investigate the influence of TRPM4 deficiency in a more complex genetic background. In a previous study by Uhl et al., TRPM4-deficient 129B6F1 hybrids were used, which under β-adrenergic stimulation with isoproterenol achieved an increased positive inotropic response in isolated left ventricular papillary muscles compared to wild-type 129B6F1 hybrids [37]. However, it remains to be investigated whether cardiac contractility in the TRPM4-deficient 129B6F1 hybrid mouse also leads to an increased β-adrenergic positive inotropy in vivo. The extent of relevance of TRPM4 deletion may also be differently pronounced between healthy mice and different degrees of disease status. Whereas in this study the inotropic response was unaltered in Trpm4–/–(Bl/6N) mice of the C57Bl/6N background, it could be shown that the β-adrenergic reserve was preserved in Trpm4–/–(Bl/6N) mice after permanent LAD ligation and significantly higher compared to Trpm4+/+(Bl/6N) leading to an increased survival rate [12]. A potential beneficial effect of inactivating or deleting TRPM4 in other cardiac disease models in the mouse or other species has not been investigated so far.
From a translational point of view, a single isogenic mouse strain is limited in its genetic diversity and may not reflect the physiological responses of the human polymorphic population [14]. The analysis of a null mutation in multiple mouse strains offers the opportunity to increase the genetic diversity of the mouse model, which allows comparison with the broad spectrum of human genetic heterogeneity [28]. Thus, the concept of inhibiting TRPM4 channels or suppressing their expression needs to be explored in different genetically diverse populations of patients suffering from cardiac failure.
References
Bacmeister L, Segin S, Medert R, Lindner D, Freichel M, Camacho Londono JE (2019) Assessment of PEEP-ventilation and the time point of parallel-conductance determination for pressure-volume analysis under beta-adrenergic stimulation in mice. Front Cardiovasc Med 6:36. https://doi.org/10.3389/fcvm.2019.00036
Barnabei MS, Palpant NJ, Metzger JM (2010) Influence of genetic background on ex vivo and in vivo cardiac function in several commonly used inbred mouse strains. Physiol Genom 42A:103–113. https://doi.org/10.1152/physiolgenomics.00071.2010
Barrick CJ, Rojas M, Schoonhoven R, Smyth SS, Threadgill DW (2007) Cardiac response to pressure overload in 129S1/SvImJ and C57BL/6J mice: temporal- and background-dependent development of concentric left ventricular hypertrophy. Am J Physiol Heart Circ Physiol 292:H2119-2130. https://doi.org/10.1152/ajpheart.00816.2006
Bers DM (2002) Cardiac excitation-contraction coupling. Nature 415:198–205. https://doi.org/10.1038/415198a
Demion M, Bois P, Launay P, Guinamard R (2007) TRPM4, a Ca2+-activated nonselective cation channel in mouse sino-atrial node cells. Cardiovasc Res 73:531–538. https://doi.org/10.1016/j.cardiores.2006.11.023
Doetschman T (2009) Influence of genetic background on genetically engineered mouse phenotypes. Methods Mol Biol 530:423–433. https://doi.org/10.1007/978-1-59745-471-1_23
Eisner DA, Caldwell JL, Trafford AW, Hutchings DC (2020) The control of diastolic calcium in the heart: basic mechanisms and functional implications. Circ Res 126:395–412. https://doi.org/10.1161/CIRCRESAHA.119.315891
Fabiato A (1983) Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol 245:C1-14. https://doi.org/10.1152/ajpcell.1983.245.1.C1
Glower DD, Spratt JA, Snow ND, Kabas JS, Davis JW, Olsen CO, Tyson GS, Sabiston DC Jr, Rankin JS (1985) Linearity of the Frank-Starling relationship in the intact heart: the concept of preload recruitable stroke work. Circulation 71:994–1009
Jacobs G, Oosterlinck W, Dresselaers T, Geenens R, Kerselaers S, Himmelreich U, Herijgers P, Vennekens R (2015a) Enhanced β-adrenergic cardiac reserve in Trpm4-/- mice with ischemic heart failure. Cardiovasc Res 105:330–339. https://doi.org/10.1093/cvr/cvv009
Guinamard R, Chatelier A, Demion M, Potreau D, Patri S, Rahmati M, Bois P (2004) Functional characterization of a Ca(2+)-activated non-selective cation channel in human atrial cardiomyocytes. J Physiol 558:75–83. https://doi.org/10.1113/jphysiol.2004.063974
Jacobs G, Oosterlinck W, Dresselaers T, Geenens R, Kerselaers S, Himmelreich U, Herijgers P, Vennekens R (2015b) Enhanced beta-adrenergic cardiac reserve in Trpm4(-)/(-) mice with ischaemic heart failure. Cardiovasc Res 105:330–339. https://doi.org/10.1093/cvr/cvv009
Jelinek M, Wallach C, Ehmke H, Schwoerer AP (2018) Genetic background dominates the susceptibility to ventricular arrhythmias in a murine model of beta-adrenergic stimulation. Sci Rep 8:2312. https://doi.org/10.1038/s41598-018-20792-5
Justice MJ, Dhillon P (2016) Using the mouse to model human disease: increasing validity and reproducibility. Dis Model Mech 9:101–103. https://doi.org/10.1242/dmm.024547
Kamp TJ, Hell JW (2000) Regulation of cardiac L-type calcium channels by protein kinase A and protein kinase C. Circ Res 87:1095–1102. https://doi.org/10.1161/01.res.87.12.1095
Kruse M, Schulze-Bahr E, Corfield V, Beckmann A, Stallmeyer B, Kurtbay G, Ohmert I, Schulze-Bahr E, Brink P, Pongs O (2009) Impaired endocytosis of the ion channel TRPM4 is associated with human progressive familial heart block type I. J Clin Invest 119:2737–2744. https://doi.org/10.1172/JCI38292
Launay P, Fleig A, Perraud AL, Scharenberg AM, Penner R, Kinet JP (2002) TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell 109:397–407. https://doi.org/10.1016/s0092-8674(02)00719-5
Liu H, El Zein L, Kruse M, Guinamard R, Beckmann A, Bozio A, Kurtbay G, Megarbane A, Ohmert I, Blaysat G, Villain E, Pongs O, Bouvagnet P (2010) Gain-of-function mutations in TRPM4 cause autosomal dominant isolated cardiac conduction disease. Circ Cardiovasc Genet 3:374–385. https://doi.org/10.1161/CIRCGENETICS.109.930867
Lum C, Shesely EG, Potter DL, Beierwaltes WH (2004) Cardiovascular and renal phenotype in mice with one or two renin genes. Hypertension 43:79–86. https://doi.org/10.1161/01.HYP.0000107401.72456.50
Madamanchi A (2007) Beta-adrenergic receptor signaling in cardiac function and heart failure. Mcgill J Med 10:99–104
Mathar I, Kecskes M, Van der Mieren G, Jacobs G, Camacho Londono JE, Uhl S, Flockerzi V, Voets T, Freichel M, Nilius B, Herijgers P, Vennekens R (2014) Increased beta-adrenergic inotropy in ventricular myocardium from Trpm4-/- mice. Circ Res 114:283–294. https://doi.org/10.1161/CIRCRESAHA.114.302835
Mathar I, Vennekens R, Meissner M, Kees F, Van der Mieren G, Camacho Londono JE, Uhl S, Voets T, Hummel B, van den Bergh A, Herijgers P, Nilius B, Flockerzi V, Schweda F, Freichel M (2010) Increased catecholamine secretion contributes to hypertension in TRPM4-deficient mice. J Clin Invest 120:3267–3279. https://doi.org/10.1172/JCI41348
Meissner M, Weissgerber P, Londono JE, Prenen J, Link S, Ruppenthal S, Molkentin JD, Lipp P, Nilius B, Freichel M, Flockerzi V (2011) Moderate calcium channel dysfunction in adult mice with inducible cardiomyocyte-specific excision of the cacnb2 gene. J Biol Chem 286:15875–15882. https://doi.org/10.1074/jbc.M111.227819
Mouse Genome Sequencing C, Waterston RH, Lindblad-Toh K, Birney E, Rogers J, Abril JF, Agarwal P, Agarwala R, Ainscough R, Alexandersson M, An P, Antonarakis SE, Attwood J, Baertsch R, Bailey J, Barlow K, Beck S, Berry E, Birren B, Bloom T, Bork P, Botcherby M, Bray N, Brent MR, Brown DG, Brown SD, Bult C, Burton J, Butler J, Campbell RD, Carninci P, Cawley S, Chiaromonte F, Chinwalla AT, Church DM, Clamp M, Clee C, Collins FS, Cook LL, Copley RR, Coulson A, Couronne O, Cuff J, Curwen V, Cutts T, Daly M, David R, Davies J, Delehaunty KD, Deri J, Dermitzakis ET, Dewey C, Dickens NJ, Diekhans M, Dodge S, Dubchak I, Dunn DM, Eddy SR, Elnitski L, Emes RD, Eswara P, Eyras E, Felsenfeld A, Fewell GA, Flicek P, Foley K, Frankel WN, Fulton LA, Fulton RS, Furey TS, Gage D, Gibbs RA, Glusman G, Gnerre S, Goldman N, Goodstadt L, Grafham D, Graves TA, Green ED, Gregory S, Guigo R, Guyer M, Hardison RC, Haussler D, Hayashizaki Y, Hillier LW, Hinrichs A, Hlavina W, Holzer T, Hsu F, Hua A, Hubbard T, Hunt A, Jackson I, Jaffe DB, Johnson LS, Jones M, Jones TA, Joy A, Kamal M et al (2002) Initial sequencing and comparative analysis of the mouse genome. Nature 420:520–562. https://doi.org/10.1038/nature01262
Nickel AG, von Hardenberg A, Hohl M, Loffler JR, Kohlhaas M, Becker J, Reil JC, Kazakov A, Bonnekoh J, Stadelmaier M, Puhl SL, Wagner M, Bogeski I, Cortassa S, Kappl R, Pasieka B, Lafontaine M, Lancaster CR, Blacker TS, Hall AR, Duchen MR, Kastner L, Lipp P, Zeller T, Muller C, Knopp A, Laufs U, Bohm M, Hoth M, Maack C (2015) Reversal of mitochondrial transhydrogenase causes oxidative stress in heart failure. Cell Metab 22:472–484. https://doi.org/10.1016/j.cmet.2015.07.008
Nilius B, Prenen J, Droogmans G, Voets T, Vennekens R, Freichel M, Wissenbach U, Flockerzi V (2003) Voltage dependence of the Ca2+-activated cation channel TRPM4. J Biol Chem 278:30813–30820. https://doi.org/10.1074/jbc.M305127200
Oosterlinck W, Vanderper A, Flameng W, Herijgers P (2011) Glucose tolerance and left ventricular pressure-volume relationships in frequently used mouse strains. J Biomed Biotechnol 2011:281312. https://doi.org/10.1155/2011/281312
Rivera J, Tessarollo L (2008) Genetic background and the dilemma of translating mouse studies to humans. Immunity 28:1–4. https://doi.org/10.1016/j.immuni.2007.12.008
Saucerman JJ, McCulloch AD (2006) Cardiac beta-adrenergic signaling: from subcellular microdomains to heart failure. Ann N Y Acad Sci 1080:348–361. https://doi.org/10.1196/annals.1380.026
Schlager G (1966) Systolic blood pressure in eight inbred strains of mice. Nature 212:519–520
Shah AP, Siedlecka U, Gandhi A, Navaratnarajah M, Al-Saud SA, Yacoub MH, Terracciano CM (2010) Genetic background affects function and intracellular calcium regulation of mouse hearts. Cardiovasc Res 87:683–693. https://doi.org/10.1093/cvr/cvq111
Shan J, Kushnir A, Betzenhauser MJ, Reiken S, Li J, Lehnart SE, Lindegger N, Mongillo M, Mohler PJ, Marks AR (2010) Phosphorylation of the ryanodine receptor mediates the cardiac fight or flight response in mice. J Clin Invest 120:4388–4398. https://doi.org/10.1172/JCI32726
Sittig LJ, Carbonetto P, Engel KA, Krauss KS, Barrios-Camacho CM, Palmer AA (2016) Genetic background limits generalizability of genotype-phenotype relationships. Neuron 91:1253–1259. https://doi.org/10.1016/j.neuron.2016.08.013
Stallmeyer B, Zumhagen S, Denjoy I, Duthoit G, Hebert JL, Ferrer X, Maugenre S, Schmitz W, Kirchhefer U, Schulze-Bahr E, Guicheney P, Schulze-Bahr E (2012) Mutational spectrum in the Ca(2+)–activated cation channel gene TRPM4 in patients with cardiac conductance disturbances. Hum Mutat 33:109–117. https://doi.org/10.1002/humu.21599
Stull LB, Hiranandani N, Kelley MA, Leppo MK, Marban E, Janssen PM (2006) Murine strain differences in contractile function are temperature- and frequency-dependent. Pflugers Arch 452:140–145. https://doi.org/10.1007/s00424-005-0020-y
Takefuji M, Wirth A, Lukasova M, Takefuji S, Boettger T, Braun T, Althoff T, Offermanns S, Wettschureck N (2012) G(13)-mediated signaling pathway is required for pressure overload-induced cardiac remodeling and heart failure. Circulation 126:1972–1982. https://doi.org/10.1161/CIRCULATIONAHA.112.109256
Uhl S, Mathar I, Vennekens R, Freichel M (2014) Adenylyl cyclase-mediated effects contribute to increased Isoprenaline-induced cardiac contractility in TRPM4-deficient mice. J Mol Cell Cardiol 74C:307–317. https://doi.org/10.1016/j.yjmcc.2014.06.007
van den Borne SW, van de Schans VA, Strzelecka AE, Vervoort-Peters HT, Lijnen PM, Cleutjens JP, Smits JF, Daemen MJ, Janssen BJ, Blankesteijn WM (2009) Mouse strain determines the outcome of wound healing after myocardial infarction. Cardiovasc Res 84:273–282. https://doi.org/10.1093/cvr/cvp207
Vennekens R, Olausson J, Meissner M, Bloch W, Mathar I, Philipp SE, Schmitz F, Weissgerber P, Nilius B, Flockerzi V, Freichel M (2007) Increased IgE-dependent mast cell activation and anaphylactic responses in mice lacking the calcium-activated nonselective cation channel TRPM4. Nat Immunol 8:312–320. https://doi.org/10.1038/ni1441
Vignier N, Mougenot N, Bonne G, Muchir A (2019) Effect of genetic background on the cardiac phenotype in a mouse model of Emery-Dreifuss muscular dystrophy. Biochem Biophys Rep 19:100664. https://doi.org/10.1016/j.bbrep.2019.100664
Waters SB, Diak DM, Zuckermann M, Goldspink PH, Leoni L, Roman BB (2013) Genetic background influences adaptation to cardiac hypertrophy and Ca(2+) handling gene expression. Front Physiol 4:11. https://doi.org/10.3389/fphys.2013.00011
Weissgerber P, Held B, Bloch W, Kaestner L, Chien KR, Fleischmann BK, Lipp P, Flockerzi V, Freichel M (2006) Reduced cardiac L-type Ca2+ current in Ca(V)beta2-/- embryos impairs cardiac development and contraction with secondary defects in vascular maturation. Circ Res 99:749–757. https://doi.org/10.1161/01.RES.0000243978.15182.c1
Acknowledgements
We are thankful to Veit Flockerzi (Saarland University, Germany) who provided the anti-TRPM4 and anti-Cavβ2 antibodies. We are thankful to Stefan Offermanns (Max-Planck-Institute for Heart and Lung Research, Germany) who kindly provided the αMHC/CreERT2 mice, and to Christin Richter, Manuela Ritzal, Hans-Peter Gensheimer and the team from the Interfakultäre Biomedizinische Forschungseinrichtung (IBF) from the Heidelberg University for expert technical assistance.
Funding
Open Access funding enabled and organized by Projekt DEAL.. This research was funded by the DZHK (German Centre for Cardiovascular Research); the BMBF (German Ministry of Education and Research); the Baden-Württemberg federal state Innovation fond; the Transregional Collaborative Research Centre-152 (TRR- 152); the collaborative Research Center 1118 (SFB 1118); the Vlaams Instituut voor Biotechnologie (VIB); Fonds voor Wetenschappelijk Onderzoek (FWO)-Vlaanderen [G.0761.10N, G.0596.12, G.0565.07, G.0966.11]; and the KULeuven Bijzonder Onderzoeksfonds [TRPLe: TRP Research Platform Leuven].
Author information
Authors and Affiliations
Contributions
Conceptualization: RM and MF; data acquisition and analysis: RM, AP and RV; writing—original draft preparation: MF, RM; writing—review and editing, all authors; visualization: RM, MF and RV; supervision: JECL, RV and MF; funding acquisition: MF, RV.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Medert, R., Pironet, A., Bacmeister, L. et al. Genetic background influences expression and function of the cation channel TRPM4 in the mouse heart. Basic Res Cardiol 115, 70 (2020). https://doi.org/10.1007/s00395-020-00831-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-020-00831-x