Abstract
Doxorubicin (DOX) is a widely used anti-tumor agent. The clinical application of the medication is limited by its side effect which can elicit myocardial apoptosis and cardiac dysfunction. However, the underlying mechanism by which DOX causes cardiomyocyte apoptosis is not clear. The aim of present study is to investigate the role of high-mobility group box 1 (HMGB1) in DOX-induced myocardial injury, and signal pathway involved in regulation of HMGB1 expression in cardiomyocytes with DOX. We found treatment of isolated cardiomyocytes and naive mice with the DOX resulted in an increased HMGB1 expression which was associated with increased myocardial cell apoptosis. Pharmacological (A-box) or genetic blockade (TLR4 deficiency, TLR4−/−) of HMGB1 attenuated the DOX-induced myocardial apoptosis and cardiac dysfunction. In addition, our study showed that DOX resulted in an increment in the generation of peroxynitrite (ONOO−) and an elevation in phosphorylation of c-Jun N terminal kinase (JNK). Pretreatment of myocytes with FeTPPS, a peroxynitrite decomposition catalyst, prevented DOX-induced JNK phosphorylation, HMGB1 expression, myocardial apoptosis and cardiac dysfunction. Genetic (JNK−/−) or pharmacological (SP600125) inhibition of JNK ameliorated the DOX-induced HMGB1 expression and diminished myocardial apoptosis and cardiac dysfunction. Taken together, our results indicate that HMGB1 mediates the myocardial injury induced by DOX and ONOO−/JNK is a key regulatory pathway of myocardial HMGB1 expression induced by DOX.







Similar content being viewed by others
References
Andrassy M, Volz HC, Igwe JC, Funke B, Eichberger SN, Kaya Z, Buss S, Autschbach F, Pleger ST, Lukic IK, Bea F, Hardt SE, Humpert PM, Bianchi ME, Mairbaurl H, Nawroth PP, Remppis A, Katus HA, Bierhaus A (2008) High-mobility group box-1 in ischemia-reperfusion injury of the heart. Circulation 117:3216–3226. doi:10.1161/CIRCULATIONAHA.108.769331
Baker CS, Dutka DP, Pagano D, Rimoldi O, Pitt M, Hall RJ, Polak JM, Bonser RS, Camici PG (2002) Immunocytochemical evidence for inducible nitric oxide synthase and cyclooxygenase-2 expression with nitrotyrosine formation in human hibernating myocardium. Basic Res Cardiol 97:409–415. doi:10.1007/s003950200050
Bianchi ME (2007) DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol 81:1–5. doi:10.1189/jlb.0306164
Bolton C, Scott GS, Smith T, Flower RJ (2008) The acute and chronic phases of chronic relapsing experimental autoimmune encephalomyelitis (CR EAE) are ameliorated by the peroxynitrite decomposition catalyst, 5,10,15,20-tetrakis(4-sulfonatophenyl)porphyrinato iron (III) chloride, (FeTPPS). Eur J Pharmacol 601:88–93. doi:10.1016/j.ejphar/2008.10.029
Davis RJ (2000) Signal transduction by the JNK group of MAP kinases. Cell 103:239–252. doi:10.1016/s0092-8674(00)00116-1
Dumitriu IE, Baruah P, Manfredi AA, Bianchi ME, Rovere-Querini P (2005) HMGB1: guiding immunity from within. Trends Immunol 26:381–387. doi:10.1016/j.it.2005.04.009
Ewer MS, Ewer SM (2010) Cardiotoxicity of anticancer treatments: what the cardiologist needs to know. Nat Rev Cardiol 7:564–575. doi:10.1038/nrcardio.2010.121
Gardella S, Andrei C, Ferrera D, Lotti LV, Torrisi MR, Bianchi ME, Rubartelli A (2002) The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep 3:995–1001. doi:10.1093/embo-reports/kvf198
Jiao XY, Gao E, Yuan Y, Wang Y, Lau WB, Koch W, Ma XL, Tao L (2009) INO-4885 [5,10,15,20-tetra[N-(benzyl-4′-carboxylate)-2-pyridinium]-21H,23H-porphine iron(III) chloride], a peroxynitrite decomposition catalyst, protects the heart against reperfusion injury in mice. J Pharmacol Exp Ther 328:777–784. doi:10.1124/jpet.108.144352
Kalyanaraman B, Joseph J, Kalivendi S, Wang S, Konorev E, Kotamraju S (2002) Doxorubicin-induced apoptosis: implications in cardiotoxicity. Mol Cell Biochem 234–235:119–124. doi:10.1023/A:1015976430790
Li Y, Arita Y, Koo HC, Davis JM, Kazzaz JA (2003) Inhibition of c-Jun N-terminal kinase pathway improves cell viability in response to oxidant injury. Am J Respir Cell Mol Biol 29:779–783. doi:10.1165/rcmb.2003-0087RC
Lotze MT, Tracey KJ (2005) High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol 5:331–342. doi:10.1038/nri1594
Loukili N, Rosenblatt-Velin N, Li J, Clerc S, Pacher P, Feihl F, Waeber B, Liaudet L (2011) Peroxynitrite induces HMGB1 release by cardiac cells in vitro and HMGB1 upregulation in the infarcted myocardium in vivo. Cardiovasc Res 89:586–594. doi:10.1093/cvr/cvq373
Michel MC, Li Y, Heusch G (2001) Mitogen-activated protein kinases in the heart. Naunyn Schmiedebergs Arch Pharmacol 363:245–266. doi:10.1007/s002100000363
Mihm MJ, Coyle CM, Schanbacher BL, Weinstein DM, Bauer JA (2001) Peroxynitrite induced nitration and inactivation of myofibrillar creatine kinase in experimental heart failure. Cardiovasc Res 49:798–807. doi:10.1016/S0008-6363(00)00307-2
Mukhopadhyay P, Rajesh M, Batkai S, Kashiwaya Y, Hasko G, Liaudet L, Szabo C, Pacher P (2009) Role of superoxide, nitric oxide, and peroxynitrite in doxorubicin-induced cell death in vivo and in vitro. Am J Physiol Heart Circ Physiol 296:H1466–H1483. doi:10.1152/ajpheart.00795.2008
Pacher P, Liaudet L, Bai P, Mabley JG, Kaminski PM, Virag L, Deb A, Szabo E, Ungvari Z, Wolin MS, Groves JT, Szabo C (2003) Potent metalloporphyrin peroxynitrite decomposition catalyst protects against the development of doxorubicin-induced cardiac dysfunction. Circulation 107:896–904. doi:10.1161/01.CIR.0000048192.52098.DD
Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, Abraham E (2004) Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem 279:7370–7377. doi:10.1074/jbc.M306793200
Rui T, Cepinskas G, Feng Q, Kvietys PR (2003) Delayed preconditioning in cardiac myocytes with respect to development of a proinflammatory phenotype: role of SOD and NOS. Cardiovasc Res 59:901–911. doi:10.1016/S0008-6363(03)00502-9
Scaffidi P, Misteli T, Bianchi ME (2002) Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418:191–195. doi:10.1038/nature00858
Shen HM, Liu ZG (2006) JNK signaling pathway is a key modulator in cell death mediated by reactive oxygen and nitrogen species. Free Radic Biol Med 40:928–939. doi:10.1016/j.freeradbiomed.2005.10.056
Singal PK, Siveski-Iliskovic N, Kaul N, Sahai M (1992) Significance of adaptation mechanisms in adriamycin induced congestive heart failure. Basic Res Cardiol 87:512–518. doi:10.1007/BF00788661
Takemura G, Fujiwara H (2007) Doxorubicin-induced cardiomyopathy from the cardiotoxic mechanisms to management. Prog Cardiovasc Dis 49:330–352. doi:10.1016/j.pcad.2006.10.002
Tsung A, Sahai R, Tanaka H, Nakao A, Fink MP, Lotze MT, Yang H, Li J, Tracey KJ, Geller DA, Billiar TR (2005) The nuclear factor HMGB1 mediates hepatic injury after murine liver ischemia-reperfusion. J Exp Med 201:1135–1143. doi:10.1084/jem.20042614
van Beijnum JR, Buurman WA, Griffioen AW (2008) Convergence and amplification of toll-like receptor (TLR) and receptor for advanced glycation end products (RAGE) signaling pathways via high mobility group B1 (HMGB1). Angiogenesis 11:91–99. doi:10.1007/s10456-008-9093-5
Volz HC, Laohachewin D, Seidel C, Lasitschka F, Keilbach K, Wienbrandt AR, Andrassy J, Bierhaus A, Kaya Z, Katus HA, Andrassy M (2012) S100A8/A9 aggravates post-ischemic heart failure through activation of RAGE-dependent NF-kappaB signaling. Basic Res Cardiol 107:1–16. doi:10.1007/s00395-012-0250-z
Volz HC, Seidel C, Laohachewin D, Kaya Z, Muller OJ, Pleger ST, Lasitschka F, Bianchi ME, Remppis A, Bierhaus A, Katus HA, Andrassy M (2010) HMGB1: the missing link between diabetes mellitus and heart failure. Basic Res Cardiol 105:805–820. doi:10.1007/s00395-010-0114-3
Xiao J, Moon M, Yan L, Nian M, Zhang Y, Liu C, Lu J, Guan H, Chen M, Jiang D, Jiang H, Liu PP, Li H (2012) Cellular FLICE-inhibitory protein protects against cardiac remodelling after myocardial infarction. Basic Res Cardiol 107:1–21. doi:10.1007/s00395-011-0239-z
Xu H, Su Z, Wu J, Yang M, Penninger JM, Martin CM, Kvietys PR, Rui T (2010) The alarmin cytokine, high mobility group box 1, is produced by viable cardiomyocytes and mediates the lipopolysaccharide-induced myocardial dysfunction via a TLR4/phosphatidylinositol 3-kinase gamma pathway. J Immunol 184:1492–1498. doi:10.4049/jimmunol.0902660
Xu H, Yao Y, Su Z, Yang Y, Kao R, Martin CM, Rui T (2011) Endogenous HMGB1 contributes to ischemia-reperfusion-induced myocardial apoptosis by potentiating the effect of TNF-α/JNK. Am J Physiol Heart Circ Physiol 300:H913–H921. doi:10.1152/ajpheart.00703.2010
Yang H, Hreggvidsdottir HS, Palmblad K, Wang H, Ochani M, Li J, Lu B, Chavan S, Rosas-Ballina M, Al-Abed Y, Akira S, Bierhaus A, Erlandsson-Harris H, Andersson U, Tracey KJ (2010) A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proc Natl Acad Sci USA 107:11942–11947. doi:10.1073/pnas.1003893107
Zhu W, Soonpaa MH, Chen H, Shen W, Payne RM, Liechty EA, Caldwell RL, Shou W, Field LJ (2009) Acute doxorubicin cardiotoxicity is associated with p53-induced inhibition of the mammalian target of rapamycin pathway. Circulation 119:99–106. doi:10.1161/CIRCULATIONAHA.108.799700
Acknowledgments
The study was supported by Grants from heart and stroke foundation of Ontario (NA-6316) and the Jiangsu Provincial Foundation for Creative Talents.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
395_2012_267_MOESM1_ESM.tif
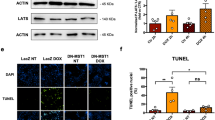
Supplementary Fig. 1 Transfection of cardiomyocytes with HMG1 shRNA plasmid resulted in a decrease in cardiomyocyte HMGB1 and prevented the DOX-indued increase in myocyte HMGB1. Cardiomyocytes were transfected with HMG1 shRNA plasmid or shRNA negative control plasmid. Subsequently, the myocytes were incubated with medium with or without doxorubicin (DOX, 0.5 µM). The HMGB1 levels in cardiomyocytes were evaluated with Western blot. Transfection cardiomyocytes with the HMG1 shRNA plasmid inhibited HMGB1 expression in cardiomyocytes. n=3. *P < 0.05 compared with negative control without DOX group, # P < 0.05 compared with negative control+DOX group. (TIFF 44693 kb)
395_2012_267_MOESM2_ESM.jpg
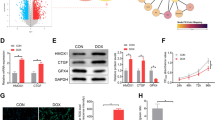
Supplementary Fig.2 Increase in peroxynitrite has been detected in myocytes deficient in JNK1 and myocardium of JNK deficient mice. a. Cardiomyocytes derived from wild type or JNK1-/- mice were treated with DOX (0.5 μM), the control myocytes were treated with vehicle. Myocyte peroxynitrite production was measures with DHR123. The peroxynitrite production was no statistical difference between wild type myocytes and JNK1-/- myocytes after DOX treatment. n=3, *P<0.05 compared to respective control; b. Wild type and JNK1-/- mice were administrated with DOX (10 mg/kg) or vehicle. Mouse hearts were harvested 5 days after the DOX and myocardial peroxynitrite was determined with Western blot by antibody againsts nitrotyrosine. The myocardial nitrotyrosine was no statistical difference between myocardium of wild type mice and those of JNK1-/- mice after DOX treatment. n=5, *P <0.05 compared to respective control. (JPEG 38 kb)
395_2012_267_MOESM3_ESM.jpg
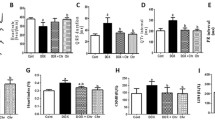
Supplementary Fig.3Wild type, TLR4-/- and JNK1-/- mice were administrated (i.p.) with either vehicle or DOX. FeTPPS (10mg/kg, i.p.) were given to DOX mice every other day starting 1 hour before the administration of DOX for inhibition of ONOO-. A-box (20mg/kg) was given to DOX mice every other days starting 4 hrs after the DOX treatment for inhibition of HMGB1. The heart rate (HR), left ventricle end systolic volume, end diastolic volume, left-ventricular end systolic pressure (LVESP), left-ventricular end diastolic pressure (LVEDP) and ±dP/dt were measured 5 days after the administration of DOX with a pressure-volume loop analysis system. n=5. *P <0.05 compared with control, #P <0.05 compared with wild type mice with DOX. (JPEG 190 kb)
Rights and permissions
About this article
Cite this article
Yao, Y., Xu, X., Zhang, G. et al. Role of HMGB1 in doxorubicin-induced myocardial apoptosis and its regulation pathway. Basic Res Cardiol 107, 267 (2012). https://doi.org/10.1007/s00395-012-0267-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-012-0267-3