Abstract
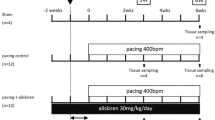
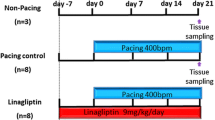
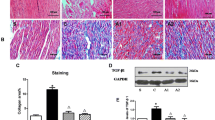
Owing to relative inefficacy and side effects of currently available antiarrhythmic drugs, current interest has shifted to treatments that target atrial fibrillation (AF) substrate. It has been suggested that calpain-induced atrial structural remodelling is under the control of renin-angiotensin system during AF. The purpose of this study is to investigate the effects of cilazapril and valsartan on the mRNA and protein expression of atrial calpains and atrial structural remodelling in AF dogs induced by chronic rapid atrial pacing. Twenty-seven dogs were randomly divided into sham-operated group (n = 6), control group (n = 7), cilazapril group (n = 7) and valsartan group (n = 7). One thin silicon plaque containing 4 pairs of electrodes was sutured to each atrium. A pacemaker was implanted in a subcutaneous pocket and attached to a screw-in epicardial lead in the right atrial appendage. The dogs in control group, cilazapril group and valsartan group were paced at 400 beats per minutes for 6 weeks. The dogs in cilazapril and valsartan groups received cilazapril (1mg · kg−· d−) or valsartan (30mg · kg−· d−) 1 week before rapid atrial pacing until pacing stop respectively. Transthoracic and transoesophageal echocardiographic examinations were performed in order to detect the changes of left atrium volume and contractile function. The inducibility and duration of AF were measured in all the groups. The expressions of atrial calpain I and calpain II mRNA were semi-quantified by reverse transcription-polymerase chain reaction. The protein levels of calpain I and calpain II in atrial myocardium were measured by Western-blot method. Pathohistological and ultrastructural changes in atrial tissue were tested by light and electron microscopy. Compared with the sham-operated control group, dramatic smaller left atrium and left atrial appendage volumes and significant higher atrial contractile function were observed in the cilazapril and valsartan groups. After 6-week atrial tachy-pacing, the mRNA and protein expressions of calpain I increased dramatically in the control group than that in the sham group, tissue calpain protein expression in all groups significantly correlated with the myolysis (r = 0.89, P < 0.01). Cilazapril and valsartan could significantly inhibit the gene and protein expressions of calpain I. No differences were found in the expression of calpain II mRNA and protein between the groups. Compared with atrial myocytes obtained from sham dogs, atrial myocytes from the control group dogs showed a reduced number of sarcomeres, a significant higher myolytic area of atria (24.3% vs. 3.1%, P < 0.01), increased vacuolization and dissolution. Cilazapril and valsartan could effectively prevent the pathohistological and ultrastructural changes induced by chronic rapid atrial pacing, dramatically decrease the area of myolysis (P < 0.05) and significantly reduce the inducibility and duration of AF. The expression of calpain I mRNA and protein increased remarkably in AF dogs. Cilazapril and valsartan can inhibit calpain I up-regulation, suppress atrial structural remodeling, and prevent the induction and promotion of AF in chronic rapid atrial pacing dogs.
Similar content being viewed by others
References
Aime-Sempe C, Folliguet T, Rucker- Martin C, Krajewska M, Krajewska S, Heimburger M, Aubier M, Mercadier JJ, Reed JC, Hatem SN (1999) Myocardial cell death in fibrillating and dilated human right atria. J Am Coll Cardiol 34:1577–1586
Ak K, Akgun S, Tecimer T, Isbir CS, Civelek A, Tekeli A, Arsan S, Cobanoglu A (2005) Determination of histopathologic risk factors for postoperative atrial fibrillation in cardiac surgery. Ann Thorac Surg 79:1970–1975
Allessie M, Ausma J, Schotten U (2002) Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc Res 54:230–246
Ausma J, Van der Velden H, Lenders MH, Duimel H, Borgers M, Allessie MA (2001) Partial recovery from structural atrial remodeling after prolonged atrial fibrillation. Circulation 104 (Suppl II):II/77
Ausma J, Wijffels M, Thone F, Wouters L, Allessie M, Borgers M (1997) Structural changes of atrial myocardium due to sustained atrial fibrillation in the goat. Circulation 96:3157–3163
Brundel BJ, Ausma J, van Gelder IC, Van der Want JJ, van Gilst WH, Crijns HJ, Henning RH (2002) Activation of proteolysis by calpains and structural changes in human paroxysmal and persistent atrial fibrillation. Cardiovasc Res 54:315–324
Brundel BJ, Kampinga HH, Henning RH (2004) Calpain inhibition prevents pacing-induced cellular remodeling in a HL-1 myocyte model for atrial fibrillation. Cardiovasc Res 62:521–328
Contard F, Koteliansky V, Marotte F, Dubus I, Rappaport L, Samuel JL (1991) Specific alterations in the distribution of extracellular matrix components within rat myocardium during the development of pressure overload. Lab Invest 64:65–75
Dai Y, Wang X, Cao L, Wu T (2004) Expression of extracellular signal-regulated kinase and angiotensin-converting enzyme in human atria during atrial fibrillation. J Huazhong Univ Sci Technolog Med Sci 24:32–36
Everett TH 4th, Li H, Mangrum JM, McRury ID, Mitchell MA, Redick JA, Haines DE (2000) Electrical, morphological, and ultrastructural remodeling and reverse remodeling in a canine model of chronic atrial fibrillation. Circulation 102:1454–1460
Fogari R, Mugellini A, Destro M, Corradi L, Zoppi A, Fogari E, Rinaldi A (2006) Losartan and prevention of atrial fibrillation recurrence in hypertensive patients. J Cardiovasc Pharmacol 47:46–50
Frustaci A, Chimenti C, Bellocci F, Morgante E, Russo MA, Maseri A (1997) Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation 96:1180–1184
Gil-Parrado S, Fernandez-Montalvan A, Assfalg-Machleidt I, Popp O, Bestvater F, Holloschi A, Knoch TA, Auerswald EA, Welsh K, Reed JC, Fritz H, Fuentes- Prior P, Spiess E, Salvesen GS, Machleidt W (2002) Ionomycin-activated calpain triggers apoptosis. A probable role for Bcl-2 family members. J Biol Chem 227:27217–27226
Godtfredsen J (1975) Atrial fibrillation Etiology, Course and Prognosis. A Follow- up Study of 1212 Cases. Copenhagen: University of Copenhagen
Goette A, Arndt M, Rocken C, Staack T, Bechtloff R, Reinhold D, Huth C, Ansorge S, Klein HU, Lendeckel U (2002) Calpains and cytokines in fibrillating human atria. Am J Physiol Heart Circ Physiol 283:H264–H272
Grishko V, Pastukh V, Solodushko V, Gillespie M, Azuma J, Schaffer S (2003) Apoptotic cascade initiated by angiotensin II in neonatal cardiomyocytes: role of DNA damage. Am J Physiol Heart Circ Physiol 285:H2364–H2372
Hirayama Y, Atarashi H, Kobayashi Y, Horie T, Iwasaki Y, Maruyama M, Miyauchi Y, Ohara T, Yashima M, Takano T (2005) Angiotensin-converting enzyme inhibitor therapy inhibits the progression from paroxysmal atrial fibrillation to chronic atrial fibrillation. Circ J 69:671–676
Kinebuchi O, Mitamura H, Shiroshita-Takeshita A, Kurita Y, Ieda M, Ohashi N, Fukuda Y, Sato T, Miyoshi S, Hara M, Takatsuki S, Nagumo M, Ogawa S (2004) Oral verapamil attenuates the progression of pacing-induced electrical and mechanical remodeling of the atrium. Circ J 68:494–500
Kossmehl P, Kurth E, Faramarzi S, Habighorst B, Shakibaei M, Wehland M, Kreutz R, Infanger M, J Danser AH, Grosse J, Paul M, Grimm D (2006) Mechanisms of apoptosis after ischemia and reperfusion: role of the renin-angiotensin system. Apoptosis 11:347–358
Li Y, Li WM, Xue JY, Han W, Yang SS, Gu HY (2004) An experimental study on the effects of cilazapril on atrial remodeling in atrial fibrillation dogs. Zhonghua Xin Xue Guan Bing Za Zhi 32:1033
Lukkarinen HP, Laine J, Aho H, Zagariya A, Vidyasagar D, Kaapa PO (2005) Angiotensin II receptor inhibition prevents pneumocyte apoptosis in surfactant-depleted rat lungs. Pediatr Pulmonol 39:349–358
Manning WJ, Silverman DI, Katz SE, Riley MF, Doherty RM, Munson JT, Douglas PS (1995) Temporal dependence of the return of atrial mechanical function on the mode of cardioversion of atrial fibrillation to sinus rhythm. Am J Cardiol 75:624–626
Nakashima H, Kumagai K, Urata H, Gondo N, Ideishi M, Arakawa K (2000) Angiotensin II antagonist prevents electrical remodeling in atrial fibrillation. Circulation 101:2612–2617
Pedersen OD, Bagger H, Kober L, Torp-Pedersen C (1999) Trandolapril reduces the incidence of atrial fibrillation after acute myocardial infarction in patients with left ventricular dysfunction. Circulation 100:376–380
Sandmann S, Yu M, Unger T (2001) Transcriptional and translational regulation of calpain in the rat heart after myocardial infarction — effects of AT(1) and AT(2) receptor antagonists and ACE inhibitor. Br J Pharmacol 132:767–777
Schotten U, Ausma J, Stellbrink C, Sabatschus I, Vogel M, Frechen D, Schoendube F, Hanrath P, Allessie MA (2001) Cellular mechanisms of depressed atrial contractility in patients with chronic atrial fibrillation. Circulation 103:691–698
Schotten U, de Haan S, Neuberger HR, Eijsbouts S, Blaauw Y, Tieleman R, Allessie M (2004) Loss of atrial contractility is primary cause of atrial dilatation during first days of atrial fibrillation. Am J Physiol Heart Circ Physiol 5:H2324–H2331
Sharma AK, Rohrer B (2004) Calciuminduced calpain mediates apoptosis via caspase-3 in a mouse photoreceptor cell line. J Biol Chem 279:35564–35572
Shi Y, Ducharme A, Li D, Gaspo R, Nattel S, Tardif JC (2001) Remodeling of atrial dimensions and emptying function in canine models of atrial fibrillation. Cardiovasc Res 52:217–225
Thijssen VL, Ausma J, Liu GS, Allessie MA, van Eys GJ, Borgers M (2000) Structural changes of atrial myocardium during chronic atrial fibrillation. Cardiovasc Pathol 9:17–28
Touyz RM, Sventek P, Lariviere R, Thibault G, Fareh J, Reudelhuber T, Schiffrin EL (1996) Cytosolic calcium changes induced by angiotensin II in neonatal rat atrial and ventricular cardiomyocytes are mediated via angiotensin II subtype 1 receptors. Hypertension 27:1090–1096
Wang KK (2000) Calpain and caspase: can you tell the difference? Trends Neurosci 23:20–26
Willems R, Sipido KR, Holemans P, Ector H, Van de Werf F, Heidbuchel H (2001) Different patterns of angiotensin II and atrial natriuretic peptide secretion in a sheep model of atrial fibrillation. J Cardiovasc Electrophysiol 12:1387–1392
Yue L, Feng J, Gaspo R, Li GR,Wang Z, Nattel S (1997) Ionic remodeling underlying action potential changes in a canine model of atrial fibrillation. Circ Res 81:512–525
Yu WC, Lee SH, Tai CT, Tsai CF, Hsieh MH, Chen CC, Ding YA, Chang MS, Chen SA (1999) Reversal of atrial electrical remodeling following cardioversion of long-standing atrial fibrillation in man. Cardiovasc Res 42:470–476
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, Y., Li, W., Gong, Y. et al. The effects of cilazapril and valsartan on the mRNA and protein expressions of atrial calpains and atrial structural remodeling in atrial fibrillation dogs. Basic Res Cardiol 102, 245–256 (2007). https://doi.org/10.1007/s00395-007-0641-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00395-007-0641-8