Abstract
Purpose
The present study aimed to evaluate the effect of chia flour associated with a high fat diet on intestinal health in female ovariectomized Wistar rats.
Methods
The study was conducted with 32 adult female ovariectomized Wistar rats, which were separated into four groups: standard diet (ST), standard diet with chia (STC), high fat diet (HF) and high fat diet with chia (HFC) for 18 weeks. Cecum content pH, short chain fatty acid content, brush border membrane functionality and morphology and the gut microbiota were evaluated.
Results
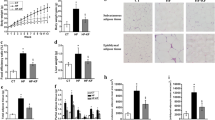
This study demonstrated that the consumption of chia flour increased the production of acetic and butyric acids, the longitudinal and circular muscle layers and crypt thickness. It also improved the expression of aminopeptidase (AP) and sucrose-isomaltase (SI) and decreased the cecum content pH. Further, the consumption of chia improved richness and decreased diversity of the microbiota. Operational Taxonomic Units (OTUs) clustering indicated difference between the ST and STC groups. In the linear discriminant analysis effect size (LEfSe) analysis, the Bacteroides genus and members of the Muribaculaceae and Lachnospiraceae families were enriched in the STC treatment group. The STC group demonstrated the enrichment of Kyoto Encyclopedia of Genes and Genomes (KEGG) metabolic pathways related to peptidoglycan and coenzyme A biosynthesis.
Conclusion
Our results suggest that chia flour, which is rich in dietary fiber and phenolic compounds, presented potential properties to improve intestinal health.








Similar content being viewed by others
References
Sun X, Zhu M-J (2017) AMP-activated protein kinase: a herapeutic target in intestinal diseases. Open Biol. https://doi.org/10.1098/rsob.170104
Tessitore A, Mastroiaco V, Vetuschi A et al (2017) Development of hepatocellular cancer induced by long term low fat-high carbohydrate diet in a NAFLD/NASH mouse model. Oncotarget 8:53482–53494. https://doi.org/10.18632/oncotarget.18585
Wali JA, Jarzebska N, Raubenheimer D et al (2020) Cardio-metabolic effects of high-fat diets and their underlying mechanisms — a narrative review. Nutrients 12:1–18
Sferra R, Pompili S, Cappariello A et al (2021) Prolonged chronic consumption of a high fat with sucrose diet alters the morphology of the small intestine. Int J Mol Sci 22:7280. https://doi.org/10.3390/ijms22147280
Abulizi N, Quin C, Brown K et al (2019) Gut mucosal proteins and bacteriome are shaped by the saturation index of dietary lipids. Nutrients 11:1–24. https://doi.org/10.3390/nu11020418
Shieh A, Epeldegui M, Karlamangla AS, Greendale GA (2020) Gut permeability, inflammation, and bone density across the menopause transition. JCI Insight 5:1–12. https://doi.org/10.1172/jci.insight.134092
Schreurs MPH, de Romano PJ, V van SA, Werner HMJ, (2021) How the gut microbiome links to menopause and obesity, with possible implications for endometrial cancer development. J Clin Med. https://doi.org/10.3390/jcm10132916
Garcia-Villatoro EL, Allred CD (2021) Estrogen receptor actions in colitis. Essays Biochem 65:1003–1013. https://doi.org/10.1042/EBC20210010
Mishima MDV, Pereira B, Lopes RC et al (2020) Bioavailability of calcium from chia (Salvia hispanica L.) in ovariectomized rats fed a high fat diet. J Am Coll Nutr. https://doi.org/10.1080/07315724.2020.1790441
Kulczynski B, Kobus-Cisowska J, Taczanowski M et al (2019) The chemical composition and nutritional value of chia seeds – current state of knowledge. Nutrients 115:1–16
Da Silva BP, Anunciação PC, da Silva Matyelka JC et al (2017) Chemical composition of Brazilian chia seeds grown in different places. Food Chem 221:1709–1716. https://doi.org/10.1016/j.foodchem.2016.10.115
Da Silva BP, Kolba N, Martino HSD et al (2019) Soluble extracts from chia seed (Salvia hispanica L.) affect brush border membrane functionality, morphology and intestinal bacterial populations in vivo (Gallus gallus). Nutrients. https://doi.org/10.3390/nu11102457
Da Silva BP, Toledo RCL, Grancieri M et al (2019) Effects of chia (Salvia hispanica L.) on calcium bioavailability and inflammation in Wistar rats. Food Res Int 116:592–599. https://doi.org/10.1016/j.foodres.2018.08.078
da Silva BP, Toledo RCL, Mishima MDV et al (2019) Effects of chia (Salvia hispanica L.) on oxidative stress and inflammation in ovariectomized adult female Wistar rats. Food Funct. https://doi.org/10.1039/C9FO00862D
da Silva BP, Dias DM, de Castro Moreira ME et al (2016) Chia seed shows good protein quality, hypoglycemic effect and improves the lipid profile and liver and intestinal morphology of Wistar rats. Plant Foods Hum Nutr 71:225–230. https://doi.org/10.1007/s11130-016-0543-8
Mishima MDV, Ladeira LCM, da Silva BP et al (2021) Cardioprotective action of chia (Salvia hispanica L.) in ovariectomized rats fed a high fat diet. Food Funct 12:3069–3082. https://doi.org/10.1039/d0fo03206a
Ayaz A, Akyol A, Inan-eroglu E et al (2017) Chia seeds added yogurt reduces short-term and increased satiety. Nutr Res Pract 11:412–418
Silva LDA, Verneque BJF, Mota APL, Duarte CK (2021) Chia seed (Salvia hispanica L.) consumption and lipid profile: a systematic review and meta-analysis. Food Funct 12:8835–8849. https://doi.org/10.1039/d1fo01287h
Fontelles MJ, Simões MG, Almeida JCFR (2010) Metodologia da pesquisa: diretrizes para o cálculo do tamanho da amostra. Rev Paran Med 24:57–64
Reeves PG, Nielsen FH, Fahey GC (1993) AIN-93 purified diets for laboratory rodents: final report of the American institute of nutrition ad hoc writing committee on the reformulation of the AIN-76A Rodent diet. J Nutr 123:1939–1951. https://doi.org/10.1093/jn/123.11.1939
Grancieri M, Costa NMB, das Graças Vaz Tostes M et al (2017) Yacon flour (Smallanthus sonchifolius) attenuates intestinal morbidity in rats with colon cancer. J Funct Foods 37:666–675. https://doi.org/10.1016/j.jff.2017.08.039
da Graças Vaz Tostes M, Viana ML, Grancieri M et al (2014) Yacon effects in immune response and nutritional status of iron and zinc in preschool children. Nutrition 30:666–672. https://doi.org/10.1016/j.nut.2013.10.016
Bradford MM (1976) A rapid and sensitive method for the quantitation microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Siegfried VR, Ruckemmann H, Stumpf G (1984) Method for the determination of organic acids in silage by high performance liquid chromatography. Landwirtsch Forsch 37:298–304
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25:402–408. https://doi.org/10.1006/meth.2001.1262
Stevenson DM, Weimer PJ (2007) Dominance of Prevotella and low abundance of classical ruminal bacterial species in the bovine rumen revealed by relative quantification real-time PCR. Appl Microbiol Biotechnol 75:165–174. https://doi.org/10.1007/s00253-006-0802-y
Caporaso JG, Lauber CL, Walters WA et al (2011) Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci U S A 108:4516–4522. https://doi.org/10.1073/pnas.1000080107
Caporaso JG, Lauber CL, Walters WA et al (2012) Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J 6:1621–1624. https://doi.org/10.1038/ismej.2012.8
Schloss PD, Westcott SL, Ryabin T et al (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541. https://doi.org/10.1128/AEM.01541-09
Edgar RC, Haas BJ, Clemente JC et al (2011) UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27:2194–2200. https://doi.org/10.1093/bioinformatics/btr381
Quast C, Pruesse E, Yilmaz P et al (2013) The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res 41:590–596. https://doi.org/10.1093/nar/gks1219
Lozupone C, Knight R (2005) UniFrac: A new phylogenetic method for comparing microbial communities. Appl Environ Microbiol 71:8228–8235. https://doi.org/10.1128/AEM.71.12.8228-8235.2005
Tousen Y, Matsumoto Y, Nagahata Y et al (2019) Resistant starch attenuates bone loss in ovariectomised mice by regulating the intestinal microbiota and bone-marrow inflammation. Nutrients. https://doi.org/10.3390/nu11020297
Hou T, Kolba N, Glahn RP, Tako E (2017) Intra-amniotic administration (Gallus gallus) of Cicer arietinum and Lens culinaris prebiotics extracts and duck egg white peptides affects calcium status and intestinal functionality. Nutrients 9:975. https://doi.org/10.3390/nu9070785
Dias DM, Kolba N, Hart JJ et al (2019) Soluble extracts from carioca beans (Phaseolus vulgaris L.) affect the gut microbiota and iron related brush border membrane protein expression in vivo (Gallus gallus). Food Res Int 123:172–180. https://doi.org/10.1016/j.foodres.2019.04.060
Wang X, Kolba N, Liang J, Tako E (2019) Alterations in gut microflora populations and brush border functionality following intra-amniotic administration (Gallus gallus) of wheat bran prebiotic extracts. Food Funct 10:4834–4843. https://doi.org/10.1039/c9fo00836e
Plamada D, Vodnar DC (2022) Polyphenols—gut microbiota interrelationship: a transition to a new generation of prebiotics. Nutrients 14:1–27. https://doi.org/10.3390/nu14010137
Santos D, Frota EG, Vargas BK et al (2022) What is the role of phenolic compounds of yerba mate (Ilex paraguariensis) in gut microbiota? Phytochemistry 203:113341. https://doi.org/10.1016/j.phytochem.2022.113341
Corrêa TAF, Rogero MM, Hassimotto NMA, Lajolo FM (2019) The two-way polyphenols-microbiota interactions and their effects on obesity and related metabolic diseases. Front Nutr 6:1–15. https://doi.org/10.3389/fnut.2019.00188
Enes BN, de Paula Dias Moreira L, Toledo RCL et al (2020) Effect of different fractions of chia (Salvia hispanica L.) on glucose metabolism, in vivo and in vitro. J Funct Foods 71:157–164. https://doi.org/10.1016/j.jff.2020.104026
de Paula Dias Moreira L, Enes BN, de São José VPB et al (2022) Chia (Salvia hispanica L.) flour and oil ameliorate metabolic disorders in the liver of rats fed a high-fat and high fructose diet. Foods. https://doi.org/10.3390/foods11030285
Fu Y, Wang Y, Gao H et al (2021) Associations among dietary omega-3 polyunsaturated fatty acids, the gut microbiota, and intestinal immunity. Mediators Inflamm. https://doi.org/10.1155/2021/8879227
Kumar M, Pal N, Sharma P et al (2022) Omega-3 fatty acids and their interaction with the gut microbiome in the prevention and amelioration of type-2 diabetes. Nutrients. https://doi.org/10.3390/nu14091723
Sant’ Ana CT, de Amorim AD, Gava AP et al (2022) Brown and golden flaxseed reduce intestinal permeability and endotoxemia, and improve the lipid profile in perimenopausal overweight women. Int J Food Sci Nutr 73:829–840
Rinninella E, Raoul P, Cintoni M et al (2019) What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms. https://doi.org/10.3390/microorganisms7010014
Abokor AA, McDaniel GH, Golonka RM et al (2021) Immunoglobulin A, an active liaison for host-microbiota homeostasis. Microorganisms 9:1–31. https://doi.org/10.3390/microorganisms9102117
Pinart M, Dötsch A, Schlicht K et al (2022) Gut microbiome composition in obese and non-obese persons: a systematic review and meta-analysis. Nutrients 14:1–41
Makki K, Deehan EC, Walter J, Bäckhed F (2018) The impact of dietary fiber on gut microbiota in host health and disease. Cell Host Microbe 23:705–715. https://doi.org/10.1016/j.chom.2018.05.012
Lagkouvardos I, Lesker TR, Hitch TCA et al (2019) Sequence and cultivation study of Muribaculaceae reveals novel species, host preference, and functional potential of this yet undescribed family. Microbiome 7:1–15. https://doi.org/10.1186/s40168-019-0637-2
Smith BJ, Miller RA, Ericsson AC et al (2019) Changes in the gut microbiome and fermentation products concurrent with enhanced longevity in acarbose-treated mice. BMC Microbiol 19:1–16. https://doi.org/10.1186/s12866-019-1494-7
Gomes MJC, Kolba N, Agarwal N et al (2021) Modifications in the intestinal functionality, morphology and microbiome following intra-amniotic administration (Gallus gallus) of grape (Vitis vinifera) stilbenes (resveratrol and pterostilbene). Nutrients. https://doi.org/10.3390/nu13093247
Guo J, Zhang M, Wang H et al (2022) Gut microbiota and short chain fatty acids partially mediate the beneficial effects of inulin on metabolic disorders in obese ob/ob mice. J Food Biochem. https://doi.org/10.1111/jfbc.14063
de SG Olivares P, Pacheco ABF, Aranha LN et al (2021) Gut microbiota of adults with different metabolic phenotypes. Nutrition. https://doi.org/10.1016/j.nut.2021.111293
Acknowledgements
The authors are thankful to the Foundation for Research Support of Minas Gerais (FAPEMIG, Brazil, number: APQ-02183-17), for the financial support for the research; we are also grateful to the Coordination for the Improvement of Higher Education Personnel (CAPES, Brazil, grant number 88887.599144/2021-00), the National Counsel of Technological and Scientific Development (CNPq, Brazil, number: 406517/2018-5, Research Productivity’s fellowships [PQ2—grant number 310910/2020-0]), and Foundation for Research an Innovation Support of Espírito Santo (FAPES, Brazil, PRONEX—CNPq/FAPES, Public Notice 24/2018—TO 567/2018).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mishima, M.D.V., da Silva, B.P., Gomes, M.J.C. et al. Effect of chia flour associated with high fat diet on intestinal health in female ovariectomized Wistar rats. Eur J Nutr 62, 905–919 (2023). https://doi.org/10.1007/s00394-022-03043-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-022-03043-2