Abstract
Purpose
Inflammatory bowel disease (IBD) is a multifactorial chronic disease of the gastrointestinal tract. Dietary intervention in the treatment of IBD has gradually attracted more attention. In this study, amino acid-balanced diets (AABD) based on grains were developed and their influences on the regulation of IBD were investigated.
Methods
Dextran sodium sulfate (DSS)-induced acute colitis mice model was employed to evaluate the effects of AABD. Pathological symptoms, intestinal inflammation, gut barrier proteins and gut microbiota were determined after AABD intake.
Results
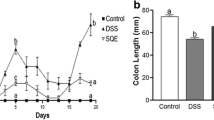
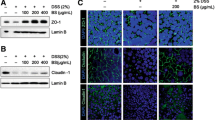
It was shown that AABD alleviated the symptoms of colitis by reducing the histological scores of mice colon, suppressing the expression of pro-inflammatory cytokines (IL-1β, IL-6 and TNF-α) and upregulating the expression of tight junction proteins. Analysis of gut microbiota revealed that AABD altered the structure of gut microbiota by decreasing the abundance and richness of harmful bacteria induced by DSS (Escherichia-Shigella, Parasutterella, etc.) and increasing that of beneficial bacteria (Akkermansia, etc.). Correlation analysis indicated that the alterations of pro-inflammatory factors were related with the change of microbiota, suggesting that the inhibitory effects of AABD on inflammation might be due to its regulation gut microbiota.
Conclusion
The AABD could efficiently mitigate colitis, and this study indicated that AABD could be applied as a promising dietary regulation strategy of IBD.






Similar content being viewed by others
References
Lobionda S, Sittipo P, Kwon HY, Lee YK (2019) The role of gut microbiota in intestinal inflammation with respect to diet and extrinsic stressors. Microorganisms 7(8):271. https://doi.org/10.3390/microorganisms7080271
Franzosa EA, Sirota-Madi A, Avila-Pacheco J, Fornelos N, Haiser HJ, Reinker S, Vatanen T, Hall AB, Mallick H, McIver LJ, Sauk JS, Wilson RG, Stevens BW, Scott JM, Pierce K, Deik AA, Bullock K, Imhann F, Porter JA, Zhernakova A, Fu J, Weersma RK, Wijmenga C, Clish CB, Vlamakis H, Huttenhower C, Xavier RJ (2019) Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat Microbiol 4(2):293–305. https://doi.org/10.1038/s41564-018-0306-4
de Souza HS, Fiocchi C (2016) Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol 13(1):13–27. https://doi.org/10.1038/nrgastro.2015.186
Li J, Zhong W, Wang W, Hu S, Yuan J, Zhang B, Hu T, Song G (2014) Ginsenoside metabolite compound K promotes recovery of dextran sulfate sodium-induced colitis and inhibits inflammatory responses by suppressing NF-kappaB activation. PLoS ONE 9(2):e87810. https://doi.org/10.1371/journal.pone.0087810
Aggeletopoulou I, Konstantakis C, Assimakopoulos SF, Triantos C (2019) The role of the gut microbiota in the treatment of inflammatory bowel diseases. Microb Pathog 137:103774. https://doi.org/10.1016/j.micpath.2019.103774
Andujar I, Recio MC, Giner RM, Cienfuegos-Jovellanos E, Laghi S, Muguerza B, Rios JL (2011) Inhibition of ulcerative colitis in mice after oral administration of a polyphenol-enriched cocoa extract is mediated by the inhibition of STAT1 and STAT3 phosphorylation in colon cells. J Agric Food Chem 59(12):6474–6483. https://doi.org/10.1021/jf2008925
Liu Y, Wang X, Chen Q, Luo L, Ma M, Xiao B, Zeng L (2020) Camellia sinensis and Litsea coreana ameliorate intestinal inflammation and modulate gut microbiota in dextran sulfate sodium-induced colitis mice. Mol Nutr Food Res 64(6):e1900943. https://doi.org/10.1002/mnfr.201900943
Kumar Singh A, Cabral C, Kumar R, Ganguly R, Kumar Rana H, Gupta A, Rosaria Lauro M, Carbone C, Reis F, Pandey AK (2019) Beneficial effects of dietary polyphenols on gut microbiota and strategies to improve delivery efficiency. Nutrients 11(9):2216. https://doi.org/10.3390/nu11092216
Zou Q, Zhang X, Liu X, Li Y, Tan Q, Dan Q, Yuan T, Liu X, Liu RH, Liu Z (2020) Ficus carica polysaccharide attenuates DSS-induced ulcerative colitis in C57BL/6 mice. Food Funct 11(7):6666–6679. https://doi.org/10.1039/d0fo01162b
Liu Y, Wang X, Hu CA (2017) Therapeutic potential of amino acids in inflammatory bowel disease. Nutrients.https://doi.org/10.3390/nu9090920
Hashimoto T, Perlot T, Rehman A, Trichereau J, Ishiguro H, Paolino M, Sigl V, Hanada T, Hanada R, Lipinski S, Wild B, Camargo SM, Singer D, Richter A, Kuba K, Fukamizu A, Schreiber S, Clevers H, Verrey F, Rosenstiel P, Penninger JM (2012) ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature 487(7408):477–481. https://doi.org/10.1038/nature11228
Guo D, Yang J, Ling F, Tu L, Li J, Chen Y, Zou K, Zhu L, Hou X (2020) Elemental diet enriched with amino acids alleviates mucosal inflammatory response and prevents colonic epithelial barrier dysfunction in mice with DSS-induced chronic colitis. J Immunol Res 2020:9430763. https://doi.org/10.1155/2020/9430763
Svolos H, Nichols Q, Ijaz, (2019) Treatment of active Crohn’s disease with an ordinary food-based diet that replicates exclusive enteral nutrition. Gastroenterology 156(5):1354–1367. https://doi.org/10.1053/j.gastro.2018.12.002
Liu W, Zhang Y, Qiu B, Fan S, Ding H, Liu Z (2018) Quinoa whole grain diet compromises the changes of gut microbiota and colonic colitis induced by dextran Sulfate sodium in C57BL/6 mice. Sci Rep 8(1):14916. https://doi.org/10.1038/s41598-018-33092-9
Zhang B, Xu Y, Liu S, Lv H, Hu Y, Wang Y, Li Z, Wang J, Ji X, Ma H, Wang X, Wang S (2020) Dietary supplementation of foxtail millet ameliorates colitis-associated colorectal cancer in mice via activation of gut receptors and suppression of the STAT3 pathway. Nutrients 12(8):2367. https://doi.org/10.3390/nu12082367
Lee S-J, Lee JH, Lee H-H, Lee S, Kim SH, Chun T, Imm J-Y (2011) Effect of mung bean ethanol extract on pro-inflammtory cytokines in LPS stimulated macrophages. Food Sci Biotechnol 20(2):519–524. https://doi.org/10.1007/s10068-011-0072-z
Liu B, Lin Q, Yang T, Zeng L, Shi L, Chen Y, Luo F (2015) Oat beta-glucan ameliorates dextran sulfate sodium (DSS)-induced ulcerative colitis in mice. Food Funct 6(11):3454–3463
Chiara MM, Franco S, Marco P, Antonio G, Donato M (2020) Nutrition, IBD and gut microbiota: a review. Nutrients 12(4):944. https://doi.org/10.3390/nu12040944
Wongkrasant P, Pongkorpsakol P, Ariyadamrongkwan J, Meesomboon R, Satitsri S, Pichyangkura R, Barrett KE, Muanprasat C (2020) A prebiotic fructo-oligosaccharide promotes tight junction assembly in intestinal epithelial cells via an AMPK-dependent pathway. Biomed Pharmacother 129:110415. https://doi.org/10.1016/j.biopha.2020.110415
Li C, Kowalski RJ, Li L, Ganjyal GM (2017) Extrusion expansion characteristics of samples of select varieties of whole yellow and green dry pea flours. Cereal Chem J 94(3):385–391. https://doi.org/10.1094/cchem-04-16-0079-r
Wirtz S, Popp V, Kindermann M, Gerlach K, Weigmann B, Fichtner-Feigl S, Neurath MF (2017) Chemically induced mouse models of acute and chronic intestinal inflammation. Nat Protoc 12(7):1295–1309. https://doi.org/10.1038/nprot.2017.044
Sun Y, Ishikawa NF, Ogawa NO, Kawahata H, Takano Y, Ohkouchi N (2020) A method for stable carbon isotope measurement of underivatized individual amino acids by multi-dimensional high-performance liquid chromatography and elemental analyzer/isotope ratio mass spectrometry. Rapid Commun Mass Spectrometry 34(20):e8885. https://doi.org/10.1002/rcm.8885
Alam MT, Amos GCA, Murphy ARJ, Murch S, Wellington EMH, Arasaradnam RP (2020) Microbial imbalance in inflammatory bowel disease patients at different taxonomic levels. Gut Pathog 12:1. https://doi.org/10.1186/s13099-019-0341-6
Martens EC, Neumann M, Desai MS (2018) Interactions of commensal and pathogenic microorganisms with the intestinal mucosal barrier. Nat Rev Microbiol 16(8):457–470. https://doi.org/10.1038/s41579-018-0036-x
Zhang H, Hu CA, Kovacs-Nolan J, Mine Y (2015) Bioactive dietary peptides and amino acids in inflammatory bowel disease. Amino Acids 47(10):2127–2141. https://doi.org/10.1007/s00726-014-1886-9
Nell S, Suerbaum S, Josenhans C (2010) The impact of the microbiota on the pathogenesis of IBD: lessons from mouse infection models. Nat Rev Microbiol 8(8):564–577. https://doi.org/10.1038/nrmicro2403
Vidal-Lletjos S, Beaumont M, Tome D, Benamouzig R, Blachier F, Lan A (2017) Dietary protein and amino acid supplementation in inflammatory bowel disease course: what impact on the colonic mucosa? Nutrients. https://doi.org/10.3390/nu9030310
Elson CO, Cong Y (2012) Host-microbiota interactions in inflammatory bowel disease. Gut Microbes 3(4):332–344. https://doi.org/10.4161/gmic.20228
El Kaoutari A, Armougom F, Gordon JI, Raoult D, Henrissat B (2013) The abundance and variety of carbohydrate-active enzymes in the human gut microbiota. Nat Rev Microbiol 11(7):497–504. https://doi.org/10.1038/nrmicro3050
Rajca S, Grondin V, Louis E, Vernier-Massouille G, Grimaud JC, Bouhnik Y, Laharie D, Dupas JL, Pillant H, Picon L, Veyrac M, Flamant M, Savoye G, Jian R, Devos M, Paintaud G, Piver E, Allez M, Mary JY, Sokol H, Colombel JF, Seksik P (2014) Alterations in the intestinal microbiome (dysbiosis) as a predictor of relapse after infliximab withdrawal in Crohn’s disease. Inflamm Bowel Dis 20(6):978–986. https://doi.org/10.1097/MIB.0000000000000036
Shin NR, Whon TW, Bae JW (2015) Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol 33(9):496–503. https://doi.org/10.1016/j.tibtech.2015.06.011
Caenepeel C, Sadat Seyed Tabib N, Vieira-Silva S, Vermeire S (2020) Review article: how the intestinal microbiota may reflect disease activity and influence therapeutic outcome in inflammatory bowel disease. Aliment Pharmacol Ther 52(9):1453–1468. https://doi.org/10.1111/apt.16096
Ankersen DV, Weimers P, Marker D, Johannesen T, Iversen S, Lilje B, Kristoffersen AB, Saboori S, Paridaens K, Skytt Andersen P, Burisch J, Munkholm P (2020) eHealth: Disease activity measures are related to the faecal gut microbiota in adult patients with ulcerative colitis. Scand J Gastroenterol 55(11):1291–1300. https://doi.org/10.1080/00365521.2020.1829031
Dong W, Huang K, Yan Y, Wan P (2020) Long-term consumption of 2-O-β-d-glucopyranosyl-l-ascorbic acid from the fruits of Lycium barbarum modulates gut microbiota in C57BL/6 mice. J Agric Food Chem 68(33):8863–8874
Zhang T, Li P, Wu X, Lu G, Marcella C, Ji X, Ji G, Zhang F (2020) Alterations of Akkermansia muciniphila in the inflammatory bowel disease patients with washed microbiota transplantation. Appl Microbiol Biotechnol 104(23):10203–10215. https://doi.org/10.1007/s00253-020-10948-7
Chen Y, Yang B, Ross RP, Jin Y, Stanton C, Zhao J, Zhang H, Chen W (2019) Orally administered CLA ameliorates DSS-induced colitis in mice via intestinal barrier improvement, oxidative stress reduction, and inflammatory cytokine and gut microbiota modulation. J Agric Food Chem 67(48):13282–13298. https://doi.org/10.1021/acs.jafc.9b05744
Zhu X, Xiang S, Feng X, Wang H, Tian S, Xu Y, Shi L, Yang L, Li M, Shen Y (2018) Impact of cyanocobalamin and methylcobalamin on inflammatory bowel disease and the intestinal microbiota composition. J Agric Food Chem 67(3):916–926
Haange SB, Jehmlich N, Hoffmann M, Weber K, Lehmann J, Von Bergen M, Slanina U (2019) Disease development is accompanied by changes in bacterial protein abundance and functions in a refined model of dextran sulfate sodium (DSS)-induced colitis. J Proteome Res 18(4):1774–1786
Sun J, Chen H, Kan J, Gou Y, Liu J, Zhang X, Wu X, Tang S, Sun R, Qian C, Zhang N, Niu F, Jin C (2020) Anti-inflammatory properties and gut microbiota modulation of an alkali-soluble polysaccharide from purple sweet potato in DSS-induced colitis mice. Int J Biol Macromol 153:708–722. https://doi.org/10.1016/j.ijbiomac.2020.03.053
Yang R, Shan S, Zhang C, Shi J, Li H, Li Z (2020) Inhibitory effects of bound polyphenol from foxtail millet bran on colitis-associated carcinogenesis by the restoration of gut microbiota in a mice model. J Agric Food Chem 68(11):3506–17
Funding
This work was supported by the National Natural Science Foundation of China [No. 31901609] and the Capacity-building project of local universities of SSTC [20060502100].
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, S., Yang, S., Zhang, Y. et al. Amino acid-balanced diets improved DSS-induced colitis by alleviating inflammation and regulating gut microbiota. Eur J Nutr 61, 3531–3543 (2022). https://doi.org/10.1007/s00394-022-02906-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-022-02906-y