Abstract
Purpose
This study investigated whether UVB-exposed wheat germ oil (WGO) is capable to improving the vitamin D status in healthy volunteers.
Methods
A randomized controlled human-intervention trial in parallel design was conducted in Jena (Germany) between February and April. Ultimately, 46 healthy males and females with low mean 25-hydroxyvitamin D (25(OH)D) levels (34.9 ± 10.6 nmol/L) were randomized into three groups receiving either no WGO oil (control, n = 14), 10 g non-exposed WGO per day (– UVB WGO, n = 16) or 10 g WGO, which was exposed for 10 min to ultraviolet B-light (UVB, intensity 500–630 µW/cm2) and provided 23.7 µg vitamin D (22.9 µg vitamin D2 and 0.89 µg vitamin D3) (+ UVB WGO, n = 16) for 6 weeks. Blood was obtained at baseline, after 3 and 6 weeks and analyzed for serum vitamin D-metabolite concentrations via LC–MS/MS.
Results
Participants who received the UVB-exposed WGO were characterized by an increase of circulating 25(OH)D2 after 3 and 6 weeks of intervention. However, the 25(OH)D3 concentrations decreased in the + UVB WGO group, while they increased in the control groups. Finally, the total 25(OH)D concentration (25(OH)D2 + 25(OH)D3) in the + UVB WGO group was lower than that of the non-WGO receiving control group after 6 weeks of treatment. In contrast, circulating vitamin D (vitamin D2 + vitamin D3) was higher in the + UVB WGO group than in the control group receiving no WGO.
Conclusion
UVB-exposed WGO containing 23.7 µg vitamin D can increase 25(OH)D2 levels but do no improve total serum levels of 25(OH)D of vitamin D-insufficient subjects.
Trial registration
ClinicalTrials.gov: NCT03499327 (registered, April 13, 2018).
Similar content being viewed by others
Introduction
Vitamin D deficiency is widespread among the population worldwide [35, 37]. In times of insufficient endogenous synthesis, e.g. by absent ultraviolet B light (UVB) exposure, recommendations for intake are 15–20 µg vitamin D per day [17, 27]. Vitamin D occurs in two forms in nature, vitamin D2 (ergocalciferol) and vitamin D3 (cholecalciferol), but food sources of vitamin D are scarce. Relevant amounts of vitamin D are only found in fatty fish [27]. Inefficient endocrine synthesis and inadequate intake of vitamin D result in suboptimal vitamin D status, which is assessed by the analysis of 25-hydroxyvitamin D (25(OH)D), a metabolite of vitamin D which is commonly used as status marker in serum or plasma [40]. Cut-off values indicating insufficient vitamin D status are discussed to be 50 nmol/L [27] or 75 nmol/L [26]. In the U.S. National Health and Nutrition Examination Survey (NHANES) study, the prevalence of individuals who have vitamin D concentrations below 50 nmol/L was 24% [38]. In Europe, the prevalence of insufficient vitamin D concentrations is even higher and is estimated to be 40.4% [9]. The intake of vitamin D supplements is one option to combat vitamin D deficiency [32, 33, 39]. However, vitamin D supplements are not widely used, at least in Germany, and therefore are not suitable to improve vitamin D status in large populations [24]. New food sources of vitamin D could be a more efficient strategy to prevent vitamin D insufficiency. The exposure of foods such as yeast, edible mushrooms or milk to UVB light is a promising approach to increase the vitamin D concentrations in foods and diets [25, 29, 32, 44]. Nowadays, UVB-exposed foods are commercially available and considered to be safe (EFSA Panel on Nutrition, Novel Foods and Food Allergens [14,15,16].
Plant oils have recently been discovered to be a potential source of vitamin D2 and vitamin D3 precursors, namely ergosterol and 7-dehydrocholesterol (7-DHC) [4]. In particular, wheat germ oil (WGO) showed relevant concentrations of ergosterol and 7-DHC, ranging from 22.1 to 34.2 µg/g and 0.638 to 0.669 µg/g, respectively. Following a 10 min– UVB-exposure (650 W/cm2, in a distance of 15 cm), the vitamin D concentrations increased from non-detectable in the non-treated oil to 1.04 µg/g vitamin D2 and 0.037 µg/g vitamin D3, respectively. It has been shown that the UVB-exposed WGO was able to significantly raise serum 25(OH)D concentrations in vitamin D-depleted mice [4]. However, data on the bioavailability of vitamin D from UVB-exposed WGO in humans are not yet available. The here presented intervention study aimed to elucidate the potential of UVB-exposed WGO in humans to improve their vitamin D status. The randomized controlled study was conducted in Jena (Germany, 51°N) during February and April in a parallel arm design. Participants who received either no WGO or non-exposed WGO served as control groups. The bioavailability of the vitamin D was assessed by measurements of 25(OH)D serum levels. Oxidation markers in the oils and blood levels of lipids and tocopherols served as safety markers or reference parameters to explain differences in plasma levels of vitamin D metabolites between the groups.
Materials and methods
Study design and wheat germ oil
The study protocol has been approved by the Ethics Committee of the Friedrich Schiller University Jena (No. 5417-01/18). The study was registered at clinicaltrials.gov (NCT03499327).
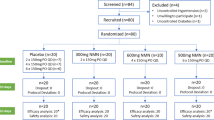
The trial was conducted as a randomized controlled study in a three-armed parallel design during February, March and April 2018, when natural sun light intensity in Jena and the surrounding region was low. The intervention period lasted 6 weeks, and the participants were scheduled to visit the study center at baseline and after 3 and 6 weeks. The participants received either no WGO (control, n = 14), non UVB-treated wheat germ oil (– UVB WGO, n = 17) or UVB-treated WGO (+ UVB WGO, n = 17) and were instructed to consume 10 g of the respective oil per day (Fig. 1). The intervention was blinded (except for the control group which received no oil), and participants were not informed about the oil they received. All investigators and physicians were unaware of the group assignment.
Flow diagram of the participants. Sixty-nine subjects were enrolled in this study. Twenty-one subjects were excluded, since they did not meet the inclusion criteria or declined to participate. Forty-eight subjects were randomized into three groups. As two subjects discontinued the intervention, 46 participants completed the 6 weeks intervention study
The WGO was acquired commercially (vomFass, Waldburg, Germany) and UVB-treated at the Martin Luther University Halle-Wittenberg under food-safe conditions. Therefore, UVB-emitting lamps (UV-15 M, Herolab, Wiesloch, Germany, analyzed intensity 500–630 µW/cm2) were placed approximately 19 cm above the oil surface (diameter of the oil surface, 3.5 mm) for 10 min. During UVB-exposure, the oil was constantly stirred by a magnetic stirrer and flushed with nitrogen to avoid oxidation. The oil was UVB-exposed 2 weeks prior to the beginning of the study and had final vitamin D2 and vitamin D3 concentrations of 2.19 ± 0.36 µg/g and 0.08 ± 0.01 µg/g (n = 9), respectively. With a consumption of 10 g oil per day, the participants met their recommended daily intake of vitamin D [27]. The non-exposed WGO was treated, except UVB exposure, in the same way as the UVB-exposed WGO and had vitamin D concentrations below the limit of quantification (LOQ, see chapter 2.4). The participants were instructed to refrigerate the oil during the study period. They were also instructed to consume the not-thermally treated oil pure or e.g., stirred in yogurt.
During each visit, anthropometric data and blood samples were collected for the determination of vitamin D metabolites (vitamin D2, vitamin D3, 25(OH)D2, 25(OH)D3), parathyroid hormone (PTH) (primary outcome measures), fatty acid distribution, lipids (triacylglycerols (TAG), total cholesterol (TC), high-density lipoprotein (HDL) cholesterol and low-density lipoprotein (LDL) cholesterol) and tocopherols (secondary outcome measures). The participants documented their normal nutritional habits over 7 days in a food frequency protocol (FFP, originated from PRODI® 5.4 software, Nutri Science, Freiburg, Germany) prior to the baseline and the 6-week visit.
Subjects
Male and female participants were recruited through newspaper advertisements, information in public institutions and personal contacts in February 2018 in Jena (Germany). Inclusion criteria were: age between 20 and 70 years and serum levels of 25(OH)D < 75 nmol/L. Exclusion criteria were: chronic diseases, medications, consumption of supplements (e.g. vitamin supplements or fish oil capsules), relevant food allergies and visits of sun beds or travel to areas with abundant UVB irradiation during or 3 months prior to the study.
Prior to the study, 69 participants were assessed for eligibility. According to the sample size calculation (see Statistical analysis), a total of 48 participants (age range, 22–66 years) met the inclusion criteria and were randomized in three groups (control, n = 14; – UVB WGO, n = 17; + UVB WGO, n = 17; Fig. 1). The participants were individually allocated to one of the three study groups, generated by a randomization list with a block size of 8. The allocation ratio of the study oil groups was 1:1.
Blood collection
After a 12-h fasting period, blood samples were drawn by venipuncture into tubes (Sarstedt, Nümbrecht, Germany). For the analysis of vitamin D metabolites and tocopherols, serum was separated by centrifugation for 10 min at 2000×g. For the analysis of PTH, fatty acids and lipids, plasma was separated by centrifugation for 10 min at 1300×g and 4 °C and aliquoted. All samples were stored at − 80 °C until further analysis.
Analytical methods
The concentration of vitamin D2 and vitamin D3 in WGO was analyzed via liquid chromatography coupled with tandem mass spectrometry (LC–MS/MS). Sample preparation was in accordance with Baur et al. [4]. Analysis was conducted with a QTRAP 5500 MS-system with ESI+ ionization (Sciex, Darmstadt, Germany) coupled to a reverse phase HPLC (Agilent 1200, Agilent Technologies, Waldbronn, Germany) equipped with a Kinetex® Phenyl-Hexyl column (100 × 2.1 mm, particle size: 2.6 µm, Phenomenex Incorporation, Torrance, CA, USA). The mobile phase consisted of (A) acetonitrile and (B) aqueous acetonitrile (1/1, v/v) with 5 mmol/L ammonium formate and 0.1% formic acid and a gradient was used for separation (0.0–2.1 min, 85% B; 2.1–7.0 min, 45% B; 18.0 min, 35% B; 22 min, 10% B; 24–26 min, 0% B; 28 min, 100% B; 28.5–30 min, 85%B) with a flow rate of 225 µL/min. MS settings and mass transitions have been reported before [5, 30]. The LOQ was 0.3 µg/g for vitamin D2 and 0.03 µg/g for vitamin D3. The fatty acid composition of the oils was performed using gas chromatography (GC-17 V3; Shimadzu, Kyoto, Japan) equipped with a flame ionization detector and an autosampler (AOC-5000), as described by Dawczynski et al.[12].
The concentrations of α-, β-, γ- and δ-tocopherol in the WGO were analyzed via LC with fluorescence detector as described before [4]. The acid value was determined according to the German official method [13]. An organoleptic characterization of the oils was conducted by a blinded panel (n = 3; ÖHMI Analytik, Magdeburg, Germany). Color, taste, flavor, and viscosity were evaluated [2]. The taste of the oil was ranked by a blinded panel (n = 3; ÖHMI Analytik), whereby the best-tasting oil was given the lowest rank (1, 2 or 3) [1]. To rule out the sole influence of the storage period of the WGO, a fresh oil was included as a control.
To analyze the serum concentrations of vitamin D2 and vitamin D3, the samples were prepared in accordance to Baur et al. [5] and were analyzed by LC–MS/MS as described above for the WGO. The serum concentrations of 25(OH)D2 and 25(OH)D3 were analyzed via the commercially available Mass Chrom® 25-OH Vitamin D3/D2 reagent kit for LC–MS/MS (Chromsystems, Gräfelfing, Germany) by use of an Agilent 1200 HPLC system and a QTRAP 5500 MS-System with APCI ionization. The LOQ was 0.25 nmol/L for vitamin D2, 1.3 nmol/L for vitamin D3, 5.3 nmol/L for 25(OH)D2 and 7.5 nmol/L for 25(OH)D3.
The concentration of PTH in plasma was analyzed via ELISA according to the manufacturer’s instruction (Immutopics Human Bioactive PTH 1–84, TECOmedical, Sissach, Switzerland).
The fatty acid distribution in plasma lipids was analyzed according to Dawczynski et al. [11] by use of gas chromatography with flame ionization detection (GC-17 V3, Shimadzu, Tokyo, Japan), equipped with a fused silica capillary column (DB-225MS, 60 m, inner diameter: 0.25 mm, film thickness: 0.25 µm, Agilent Technologies). Fatty acid concentrations were expressed as percentage of the total area of all fatty acid methyl esters (% of total fatty acid methyl esters, FAME) using GC solution software version 2.3 (Shimadzu).
Plasma lipids (TAG, TC, HDL-cholesterol, LDL-cholesterol) were measured by using an Abbott Architect CI 16,200 analyzer (Abbott, Wiesbaden, Germany) according to the manufacturer’s recommendations.
High performance LC with fluorescent detection was used to analyze the concentration of α-tocopherol in serum as described before [4].
All analyses were run in duplicate.
Statistical analysis
Sample size calculation was based on previously published data of Seibert et al. [41], who showed an increase in 25(OH)D concentrations from 38 ± 14 to 70 ± 15 nmol/L (Δ25(OH)D, 32 nmol/L; 84% rise) after 8 weeks of supplementation with 20 µg vitamin D3 daily during winter time. In contrast, 25(OH)D concentrations in the placebo-treated control group decreased from 38 ± 15 to 32 ± 14 nmol/L. Initially assuming that a daily consumption of 10 g UVB-treated WGO would provide 10 µg vitamin D per day, we hypothesized an increase of 42% to approximately 54 nmol/L in the UVB WGO group. Thus, we calculated a sample size of 14 participants per group with an effect size of 1.47, a 95% power and a significance level of 0.05 (G*Power version 3.1.9.2). To take potential dropouts in the WGO consuming groups into account, 17 participants were included in each WGO group.
Statistical analyses were conducted using SPSS statistics version 24 (IBM, Armonk, NY, USA). For all statistical tests α = 0.05 was used to decide if the test result is significant or not. If values were below the LOQ, the appropriate LOQ was used for statistical analysis. To compare the values of the three groups and the absolute changes between baseline and week 6, the data were tested for normal distribution using the Shapiro–Wilk test. Given a normal distribution for all three groups, comparison was done with Welch’s one-way analysis of variance (ANOVA) test. Individual differences were investigated with Games–Howell test. Otherwise, the Kruskal–Wallis test was used and differences between individual groups were investigated using pairwise Mann–Whitney U tests with Bonferroni correction.
In case of normal distribution (Shapiro–Wilk test) and equal variances (Mauchly's sphericity test), time-depending differences within a group were compared by repeated measurement ANOVA with post-hoc comparison by Bonferroni. Otherwise, the Friedman test was used and the P values were corrected by Bonferroni.
Results
Characterization of the wheat germ oils
Since the vitamin D concentrations in UVB-treated WGO are known to increase during storage conditions [4], the concentration of vitamin D in WGO was assessed during the study period. In the non-treated WGO, the concentration of vitamin D (vitamin D2 and vitamin D3) remained below the LOQ during the whole study period. In the UVB-treated WGO, the vitamin D2 concentration ranged from 2.06 to 2.70 µg/g (mean, 2.29 ± 0.18 µg/g, n = 9) and the vitamin D3 concentration ranged from 0.079 to 0.099 µg/g (mean, 0.089 ± 0.008 µg/g, n = 9) (Table 1).
The composition of characteristic fatty acids was similar between the non-treated and UVB-exposed WGOs and did not change during the study period (Table 1). To elucidate, whether the UVB-exposure was accompanied by an increased oxidation of the fatty acids in WGO, the acid value and the concentrations of α-, β-, γ- and δ-tocopherol were analyzed at baseline and at the end of the study period. However, no obvious time- and treatment-dependent differences were observed (Table 1). Organoleptic tests revealed UVB-light induced changes in the flavor of the WGO, because the UVB-exposed WGO achieved the highest number of points, followed by the non-exposed WGO and the fresh WGO (Table 1).
Baseline characteristics
Forty-eight subjects were enrolled in this study. The baseline characteristics are given in Table 2. Two subjects did not complete the study, due to personal reasons (dropout rate 4.2%). In total, 46 participants (age range, 22–65 years; 19 males/27 females) completed the 6-week-intervention (Fig. 1). The average baseline concentration of 25(OH)D was 35.5 ± 10.4 nmol/L. After the study, the subjects were asked for their compliance in daily oil consumption. From the 32 participants which had to consume the oil daily, eight subjects of the + UVB WGO group (50% of group total) and four subjects of the – UVB WGO group (25% of group total) admitted that they did not consume the oils during 3 or 4 days.
Nutrient intake assessed by food frequency protocols
The mean daily intake of energy, fat and PUFAs was higher in the two groups which received the WGO (data in Supplementary Table S1). The average daily vitamin D intake was 3.20 ± 2.73 µg vitamin D at baseline and 2.72 ± 2.24 µg at week 6. The vitamin D intake with the background diet (without the WGO) was not different between the groups at any time (Table S1).
Concentrations of vitamin D status markers
To elucidate the potential of UVB-exposed WGO to improve vitamin D status, the circulating serum concentrations of 25(OH)D were analyzed. At baseline, the 25(OH)D2 concentration was below the LOQ in all subjects except one (– UVB WGO group). In subjects treated with the + UVB WGO the concentration of 25(OH)D2 increased from baseline to week 3 and 6, respectively (P < 0.001 for both time points). The mean level of 25(OH)D2 in the control and – UVB WGO groups remained below the LOQ (Table 3). The concentration of 25(OH)D3 in the three groups was comparable at baseline and changed during the intervention. The 25(OH)D3 levels in the + UVB WGO group decreased steadily during the intervention period, resulting in significant lower levels after 3 and 6 weeks in the + UVB WGO group compared to both other groups (P < 0.001 for both time points, Table 3). In contrast, the control and the – UVB WGO group showed a moderate rise in their 25(OH)D3 concentrations over the study period (P < 0.001 for both groups). The higher concentration of 25(OH)D3 in the control and – UVB WGO groups compared to the + UVB WGO group resulted from unusually high 25(OH)D3 concentrations analyzed in a few individuals in these groups (three individuals in the control group and three individuals in the – UVB WGO group had 25(OH)D concentrations > 50 nmol/l), whilst other participants had mean values of 29.2 ± 11.0 nmol/L. To elucidate the net effect of the UVB-exposed WGO on vitamin D status, the total 25(OH)D concentrations were calculated by summing up 25(OH)D2 and 25(OH)D3. Data show that the total 25(OH)D concentrations in the + UVB WGO group remained unchanged, while the concentration of total 25(OH)D moderately increased in the control and in the – UVB WGO groups over the study period (P < 0.001 for both groups; Table 3). Finally, the concentration of total 25(OH)D was lower in the + UVB WGO group than in the control group.
In comparison to the 25(OH)D levels, the vitamin D concentrations in serum were noticeably lower. Vitamin D analysis revealed that the circulating concentration of vitamin D2 in the + UVB WGO group increased from baseline to week 6 of the study (P < 0.001), in contrast to the two other groups. Data on plasma vitamin D3 showed a heterogeneous picture. The concentration of vitamin D3 rose from baseline to week 6 in the two WGO groups (P < 0.001 for both groups), but not in the control group (Table 3). No difference in the final plasma vitamin D3 concentration was seen between the + UVB WGO group and the – UVB WGO group. Calculation of the total vitamin D (vitamin D2 + vitamin D3), revealed a time-dependent rise in in the – UVB WGO and in the + UVB WGO group (P < 0.001 for both groups), while the total vitamin D concentration in control group remained unchanged (Table 3). The absolute changes of the vitamin D metabolites after 6 weeks of intervention compared to baseline are given in Table 4.
To elucidate whether the vitamin D source affects the hydroxylation of vitamin D to 25(OH)D, we calculated the ratio of 25(OH)D2 to vitamin D2, of 25(OH)D3 to vitamin D3 and of total 25(OH)D to total vitamin D (Table 3). Hydroxylation of vitamin D3, and as a result of total vitamin D, was reduced by the daily consumption of both WGOs. However, this effect was much more pronounced in the + UVB WGO than in the – UVB WGO group.
In contrast to vitamin D metabolites, the PTH concentrations did not differ between the groups at any time. After 3 weeks of intervention, the PTH concentrations were higher in the control and in the – UVB WGO groups compared to baseline (P < 0.05 and P < 0.01, respectively), but not in the + UVB WGO group. However, the PTH concentrations were finally not different after 6 weeks of intervention compared to baseline in all three groups (Table 3).
Concentrations of plasma lipids
To ensure that changes in serum vitamin D concentrations were not caused by changes in plasma lipids, we analyzed the fatty acid profile, and the plasma concentrations of TAGs and cholesterol. Data show no differences in the profiles of saturated fatty acids (SFAs), monounsaturated fatty acids (MUFAs) and polyunsaturated fatty acids (PUFAs) between the groups after the intervention (Table 5). Since WGO contains high concentrations of linoleic acid (LA), the concentration of LA was analyzed as compliance marker in plasma of the subjects at baseline and after 6 weeks. The plasma LA concentration in the – UVB WGO and the + UVB WGO groups increased from baseline to week 6 of the intervention (Δ baseline vs. week 6: + 1.44 ± 2.61 and + 1.71 ± 2.45% FAME, respectively; P < 0.05), while the LA concentration in the control group slightly but not statistically significant declined (Δ: − 0.43 ± 2.22% FAME). Finally, the LA concentration did not differ between the three groups (Table 5). The concentrations of TAGs, total, HDL- and LDL-cholesterol and their ratio were similar between the groups (P < 0.1, Table 5).
To investigate whether the intake of UVB-treated WGO was accompanied by changes in plasma antioxidants, we analyzed α-tocopherols, but found no time- and group-specific differences (Table 5).
Discussion
This study investigated the efficacy of UVB-treated WGO to improve the vitamin D status of healthy subjects during the wintertime, when the endogenous vitamin D synthesis is reduced. Firstly, we were able to markedly increase the vitamin D content of WGO by the exposure of this oil to UVB light. The resulting vitamin D content of WGO, which mainly comprised vitamin D2, amounted to 2.37 ± 0.16 µg/g (n = 9), so that a daily intake of 10 g UVB-treated WGO provided 23.7 µg vitamin D. This intake can be considered as safe, although it is moderately higher (1.6-times) than the recommended daily intake for vitamin D [27]. In addition, the unchanged concentrations of circulating α-tocopherols are not indicative of any oxidative stress associated with the consumption of UVB-treated WGO. However, the UVB-exposure did negatively affect the taste of the oil, as shown by the organoleptic tests.
The major finding of the current study was that UVB-exposed WGO leads to an increase of the serum levels of 25(OH)D2, without improving the total 25(OH)D concentrations. The latter resulted from the finding that the treatment with UVB-exposed WGO had lowered 25(OH)D3 disproportionately stronger than the treatment with the unexposed WGO. There may be multiple reasons for the strong decline in 25(OH)D3 after the consumption of UVB-treated WGO. Firstly, the efficiency of a vitamin D2 to increase the serum concentrations of 25(OH)D has been shown to be lower than that of vitamin D3 (reviewed in [43]). A few studies which distinguished between the 25(OH)D2 and 25(OH)D3 concentrations found a marked reduction of 25(OH)D3 in vitamin D2 treated groups that was stronger than in groups that received no vitamin D [3, 7, 18, 32]. Although both isoforms of vitamin D are considered equally in the treatment of rickets [36], Lehman et al. found substantially lower levels of 25(OH)D in the group supplemented with vitamin D2 than in the vitamin D3 supplemented group [32]. The lower 25(OH)D levels in the vitamin D2 group were caused by a marked decline in 25(OH)D3 in comparison to the vitamin D3 group. A phenomenon, which has also been demonstrated vice versa [23]. Although the reason for the strong decline of 25(OH)D3 concentrations in response to vitamin D2 from the UVB-exposed WGO in the present study remains unclear, the data are indicative for a reduced hepatic hydroxylation of vitamin D. This could be due to a competition of vitamin D2 and vitamin D3 for 25-hydroxylase. Alternatively, the degradation of 25(OH)D3 as a result of an upregulated expression of catabolic enzymes by vitamin D2 could be enhanced. So far, three enzymes of the cytochrome P450 family (CYP) are known to 25-hydroxylate vitamin D in the liver. While CYP2R1 can hydroxylate both vitamin D isoforms at C-position 25 [46], CYP27A1 is capable of hydroxylating only vitamin D3 [21] and CYP3A4 only vitamin D2 [22]. The latter is also known to degrade vitamin D3, by mono-hydroxylation of 25(OH)D3 at several other positions, including C-positions 23, 24, 26 and in particular C4 [45]. Thus, we speculate that the vitamin D2 and D3 can activate the various hydroxylases in different ways, thereby influencing the 25(OH)D2 and 25(OH)D3 profile. Secondly, in contrast to semi-synthetic vitamin D2 supplements, the UVB treatment of WGO could have resulted in the formation of vitamin D photoisomers such as lumisterol or tachysterol, which in turn may affect the metabolism of the vitamin D3 isoforms. In accordance with the current data, UVB-treated mushrooms (providing 17.1 µg vitamin D2) were also not able to increase total 25(OH)D levels, since the 25(OH)D3 concentrations decreased by 10.3 ± 1.75 nmol/L after 3 weeks and by 20.6 ± 14.6 nmol/L after 6 weeks of intervention [42]. By that, the decrease of 25(OH)D3 was higher as the decrease in the current study, which was 8.27 ± 6.19 nmol/L and 9.40 ± 8.48 nmol/L after 3 and 6 weeks, respectively. UVB exposure of food is usually accompanied by the formation of photoisomers. Interestingly, data have shown that vitamin D photoisomers such as lumisterol may lower the circulating 25(OH)D3 concentrations in mice [31]. Thus, it is tempting to speculate that availability of vitamin D from UVB exposed food is modified by photoproducts which are synthesized during UVB irradiation. Additionally, the lack of increase in serum 25(OH)D after the consumption of vitamin D2 may result from the low dose of administered vitamin D2, because higher doses of vitamin D2 are capable of increasing total serum concentrations of 25(OH)D2. Findings show that high doses of vitamin D2 from a UVB-exposed mushrooms soup (700 µg per serving), given as a weekly bolus, can compensate the decrease in 25(OH)D3 [10, 44]. It should be pointed out that the 25(OH)D3 concentrations increased in the control and – UVB WGO groups, although the participants did not receive any vitamin D, and were encouraged to avoid direct sun exposure and to use sun protection. The observed increase in 25(OH)D3 in the control and – UVB WGO groups were indicative of an enhanced endogenous vitamin D synthesis. However, the individual 25(OH)D3 levels indicate that the high mean values of 25(OH)D3 in the control and – UVB WGO groups were caused by only a few individuals, who do not adhere to the recommendation to avoid sun exposure.
In contrast to the analysis of 25(OH)D, serum levels of vitamin D2, did not differ between the – UVB WGO and the + UVB WGO groups, and the – UVB WGO and the control groups, respectively (Table 3). Compared to the control group, the total vitamin D concentrations were higher in the two WGO groups, although the difference was only significant between the + UVB WGO and the control groups, and not between the – UVB WGO and the control groups, due to the high variances. Various compounds are able to modulate the vitamin D metabolism. These are long-chain fatty acids [19] and phytochemicals such as pinoresinol [20] which has been found to lower the intestinal absorption of vitamin D, and ergosterol that appears to increase oral vitamin D3 in plasma and liver, of mice [5]. Thus, it is tempting to speculate that the higher vitamin D3 concentrations in the – UVB WGO and the + UVB WGO groups observed in the current study are caused by the high amount of ergosterol found in the WGOs. To elucidate, whether the differences in 25(OH)D3 were attributed to differences in plasma lipids, we measured plasma fatty acids and other lipids, but found no significant differences between the groups after the intervention. Thus, we suggest that the plasma lipids were not attributable to the observed differences of vitamin D3 metabolites. Analysis of plasma PTH revealed no differences between the groups, which indicates the inability of the UVB-treated WGO to improve the vitamin D status.
Our study has several limitations. First, data assume that a few participants had produced vitamin D via the endogenous synthesis although they were asked to avoid any sun light exposure and to use sun protection. Second, the large differences in the response of vitamin D status after vitamin D intake [8, 32], would have required a higher number of study participants to clearly show treatment differences. Third, the male/female ratio was unequally distributed in the groups (control group: males, 3; females, 11; the WGO groups: males, 8; females 8). The higher number of males in the two WGO receiving groups may have resulted in the higher intake of energy, fat and polyunsaturated fatty acids assessed by the FFP [6, 34].
Conclusion
UVB exposure can significantly increase the vitamin D2 concentrations of WGO. The UVB-exposed WGO was able to increase the 25(OH)D2 levels in vitamin D insufficient healthy individuals. However, the increase in serum 25(OH)D2 was accompanied by a concurrent decrease of 25(OH)D3 levels, while the 25(OH)D3 levels increased in the control and the – UVB WGO group.
Availability of data and materials
Not applicable.
Code availability
Not applicable.
Abbreviations
- 25(OH)D:
-
25-Hydroxyvitamin D
- 7-DHC:
-
7-Dehydrocholesterol
- ANOVA:
-
Analysis of variance
- FAME:
-
Fatty acid methyl esters
- FFP:
-
Food frequency protocol
- HDL:
-
High-density lipoprotein
- LA:
-
Linoleic acid
- LDL:
-
Low-density lipoprotein
- LOQ:
-
Limit of quantification
- MUFA:
-
Monounsaturated fatty acid
- PTH:
-
Parathyroid hormone
- PUFA:
-
Polyunsaturated fatty acid
- SFA:
-
Saturated fatty acid
- TAG:
-
Triacylglycerols
- TC:
-
Total cholesterol
- UVB:
-
Ultraviolet B light
- WGO:
-
Wheat germ oil
References
Amtliche Sammlung von Untersuchungsverfahren nach § 64 LFGB (Hg.) (2011) Sensorische Prüfverfahren - Rangordnungsprüfung (nach DIN ISO 8587), L0090-4. Beuth Verlag GmbH, Berlin
Amtliche Sammlung von Untersuchungsverfahren nach § 64 LFGB (Hg.) (2015) Sensorische Prüfverfahren; Einfach beschreibende Prüfung (nach DIN 10964), L0090–6. Beuth Verlag GmbH, Berlin
Armas LAG, Hollis BW, Heaney RP (2004) Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab 89(11):S. 5387-5391. https://doi.org/10.1210/jc.2004-0360
Baur AC, Brandsch C, König B, Hirche F, Stangl GI (2016) Plant oils as potential sources of vitamin D. Front Nutr 3:S. 29. https://doi.org/10.3389/fnut.2016.00029
Baur AC, Kühn J, Brandsch C, Hirche F, Stangl GI (2019) Intake of ergosterol increases the vitamin D concentrations in serum and liver of mice. J Steroid Biochem Mol Biol 194:S. 105435. https://doi.org/10.1016/j.jsbmb.2019.105435
Bennett E, Peters SAE, Woodward M (2018) Sex differences in macronutrient intake and adherence to dietary recommendations: findings from the UK Biobank. BMJ Open 8(4):e020017. https://doi.org/10.1136/bmjopen-2017-020017
Binkley N, Gemar D, Engelke J, Gangnon R, Ramamurthy R, Krueger D, Drezner MK (2011) Evaluation of ergocalciferol or cholecalciferol dosing, 1,600 IU daily or 50,000 IU monthly in older adults. J Clin Endocrinol Metab 96(4):S. 981-988. https://doi.org/10.1210/jc.2010-0015
Carlberg C, Haq A (2018) The concept of the personal vitamin D response index. J Steroid Biochem Mol Biol 175:12–17. https://doi.org/10.1016/j.jsbmb.2016.12.011
Cashman KD, Dowling KG, Škrabáková Z, Gonzalez-Gross M, Valtueña J, de Henauw S et al (2016) Vitamin D deficiency in Europe: pandemic? Am J Clin Nutr 103(4):S. 1033-1044. https://doi.org/10.3945/ajcn.115.120873
Cashman KD, Kiely M, Seamans KM, Urbain P (2016) Effect of ultraviolet light-exposed mushrooms on vitamin D status: liquid chromatography-tandem mass spectrometry reanalysis of biobanked sera from a randomized controlled trial and a systematic review plus meta-analysis. J Nutr 146(3):S. 565-575. https://doi.org/10.3945/jn.115.223784
Dawczynski C, Massey KA, Ness C, Kiehntopf M, Stepanow S, Platzer M et al (2013) Randomized placebo-controlled intervention with n-3 LC-PUFA-supplemented yoghurt: effects on circulating eicosanoids and cardiovascular risk factors. Clin Nutr 32(5):S. 686-696. https://doi.org/10.1016/j.clnu.2012.12.010
Dawczynski C, Schubert R, Jahreis G (2007) Amino acids, fatty acids, and dietary fibre in edible seaweed products. Food Chem 103(3):S. 891-899. https://doi.org/10.1016/j.foodchem.2006.09.041
Gesellschaft D, für Fettwissenschaften e.V. (eds) (1994) Säurezahl. Wissenschaftliche Verlagsgesellschaft mbH, Stuttgart
EFSA Panel on Dietetic Products, Nutrition and Allergies (EFSA NDA Panel) (2014) Scientific Opinion on the safety of vitamin D-enriched UV-treated baker’s yeast. EFSA J. https://doi.org/10.2903/j.efsa.2014.3520
EFSA Panel on Dietetic Products, Nutrition and Allergies (EFSA NDA Panel) (2016) Safety of UV-treated milk as a novel food pursuant to regulation (EC) No 258/97. EFSA J. https://doi.org/10.2903/j.efsa.2016.4370
EFSA Panel on Nutrition, Novel Foods and Food Allergens (EFSA NDA Panel) (2020) Safety of vitamin D2 mushroom powder as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. https://doi.org/10.2903/j.efsa.2020.5948
German Nutrition Society (2012) New reference values for vitamin D. Ann Nutr Metab 60(4):S. 241-246. https://doi.org/10.1159/000337547
Glendenning P, Chew GT, Seymour HM, Gillett MJ, Goldswain PR, Inderjeeth CA et al (2009) Serum 25-hydroxyvitamin D levels in vitamin D-insufficient hip fracture patients after supplementation with ergocalciferol and cholecalciferol. Bone 45(5):S. 870-875. https://doi.org/10.1016/j.bone.2009.07.015
Goncalves A, Gleize B, Roi S, Nowicki M, Dhaussy A, Huertas A et al (2013) Fatty acids affect micellar properties and modulate vitamin D uptake and basolateral efflux in Caco-2 cells. J Nutr Biochem 24(10):S. 1751-1757. https://doi.org/10.1016/j.jnutbio.2013.03.004
Goncalves A, Margier M, Tagliaferri C, Lebecque P, Georgé S, Wittrant Y et al (2016) Pinoresinol of olive oil decreases vitamin D intestinal absorption. Food Chem 206:S. 234-238. https://doi.org/10.1016/j.foodchem.2016.03.048
Guo Y-D, Strugnell S, Back DW, Jones G (1993) Transfected human liver cytochrome P-450 hydroxylates vitamin D analogs at different side-chain positions. Proc Natl Acad Sci U S A 90:S. 8668–8672 (zuletzt geprüft am 16.03.2021)
Gupta RP, Hollis BW, Patel SB, Patrick KS, Bell NH (2004) CYP3A4 is a human microsomal vitamin D 25-hydroxylase. J Bone Miner Res 19(4):S. 680-688. https://doi.org/10.1359/JBMR.0301257
Hammami MM, Abuhdeeb K, Hammami S, Yusuf A (2019) Vitamin-D2 treatment-associated decrease in 25(OH)D3 level is a reciprocal phenomenon: a randomized controlled trial. BMC Endocr Disord 19(1):8. https://doi.org/10.1186/s12902-019-0337-8
Hintzperter B, Mensink GBM, Thierfelder W, Müller MJ, Scheidt-Nave C (2008) Vitamin D status and health correlates among German adults. Eur J Clin Nutr 62:1079–1089 (zuletzt geprüft am 13.11.2020)
Hohman EE, Martin BR, Lachcik PJ, Gordon DT, Fleet JC, Weaver CM (2011) Bioavailability and efficacy of vitamin D2 from UV-irradiated yeast in growing, vitamin D-deficient rats. J Agric Food Chem 59(6):S. 2341-2346. https://doi.org/10.1021/jf104679c
Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP et al (2011) Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96(7):1911–1930. https://doi.org/10.1210/jc.2011-0385
Institute of Medicine (IOM) (2011) Dietary reference intakes. In: Calcium, vitamin D. National Academies Press, Washington DC. https://doi.org/10.17226/13050
Jakobsen J, Jensen SK, Hymøller L, Andersen EW, Kaas P, Burild A, Jäpelt RB (2015) Short communication: artificial ultraviolet B light exposure increases vitamin D levels in cow plasma and milk. J Dairy Sci 98(9):S. 6492-6498. https://doi.org/10.3168/jds.2014-9277
Jasinghe VJ, Perera CO (2005) Distribution of ergosterol in different tissues of mushrooms and its effect on the conversion of ergosterol to vitamin D2 by UV irradiation. Food Chem 92(3):S. 541-546. https://doi.org/10.1016/j.foodchem.2004.08.022
Kiourtzidis M, Kühn J, Schutkowski A, Baur AC, Hirche F, Stangl GI (2020) Inhibition of Niemann-Pick C1-like protein 1 by ezetimibe reduces uptake of deuterium-labeled vitamin D in mice. J Steroid Biochem Mol Biol 197:S. 105504. https://doi.org/10.1016/j.jsbmb.2019.105504
Kotwan J, Baur AC, Kühn J, Stangl GI (2021) Oral intake of lumisterol affects the metabolism of vitamin D. Mol Nutr Food Res. https://doi.org/10.1002/mnfr.202001165
Lehmann U, Hirche F, Stangl GI, Hinz K, Westphal S, Dierkes J (2013) Bioavailability of vitamin D2 and D3 in healthy volunteers, a randomized placebo-controlled trial. J Clin Endocrinol Metab 98(11):4339–4345. https://doi.org/10.1210/jc.2012-4287
Manson JE, Cook NR, Lee I-M, Christen W, Bassuk SS, Mora S et al (2019) Vitamin D supplements and prevention of cancer and cardiovascular disease. N Engl J Med 380(1):33–44. https://doi.org/10.1056/NEJMoa1809944
Max-Rubner-Institut (2013) Nationale Verzehrsstudie II. Lebensmittelverzehr und Nährstoffzufuhr auf Basis von 24h-Recalls. Unter Mitarbeit von Carolin Krems, Carina Walter, Thorsten Heuer und Ingrid Hoffmann. Hg. v. Max-Rubner-Institut. Bundesforschungsinstitut für Ernährung und Lebensmittel. Karlsruhe, zuletzt geprüft am 18.11.2020.
Palacios Ca, Gonzalez L (2014) Is vitamin D deficiency a major global public health problem? J Steroid Biochem Mol Biol 144(Pt A, S.):138–145. https://doi.org/10.1016/j.jsbmb.2013.11.003
Park EA (1940) The therapy of rickets. JAMA 115(5):370–379 (zuletzt geprüft am 16.03.2021)
Roth DE, Abrams SA, Aloia J, Bergeron G, Bourassa MW, Brown KH et al (2018) Global prevalence and disease burden of vitamin D deficiency: a roadmap for action in low- and middle-income countries. Ann N Y Acad Sci 1430(1):44–79. https://doi.org/10.1111/nyas.13968
Schleicher RL, Sternberg MR, Looker AC, Yetley EA, Lacher DA, Sempos CT et al (2016) National estimates of serum total 25-hydroxyvitamin D and metabolite concentrations measured by liquid chromatography-tandem mass spectrometry in the US Population during 2007–2010. J Nutr 146(5):1051–1061. https://doi.org/10.3945/jn.115.227728
Scragg R, Stewart AW, Waayer D, Lawes CMM, Toop L, Sluyter J et al (2017) Effect of monthly high-dose vitamin d supplementation on cardiovascular disease in the vitamin D assessment study: a randomized clinical trial. JAMA Cardiol 2(6):608–616. https://doi.org/10.1001/jamacardio.2017.0175
Seamans KM, Cashman KD (2009) Existing and potentially novel functional markers of vitamin D status: a systematic review. Am J Clin Nutr 89(6):1997S-2008S. https://doi.org/10.3945/ajcn.2009.27230D
Seibert E, Lehmann U, Riedel A, Ulrich C, Hirche F, Brandsch C et al (2017) Vitamin D3 supplementation does not modify cardiovascular risk profile of adults with inadequate vitamin D status. Eur J Nutr 56(2):621–634. https://doi.org/10.1007/s00394-015-1106-8
Stephensen CB, Zerofsky M, Burnett DJ, Lin Y-P, Hammock BD, Hall LM, McHugh T (2012) Ergocalciferol from mushrooms or supplements consumed with a standard meal increases 25-hydroxyergocalciferol but decreases 25-hydroxycholecalciferol in the serum of healthy adults. J Nutr 142(7):1246–1252. https://doi.org/10.3945/jn.112.159764
Tripkovic L, Lambert H, Hart K, Smith CP, Bucca G, Penson S et al (2012) Comparison of vitamin D2 and vitamin D3 supplementation in raising serum 25-hydroxyvitamin D status: a systematic review and meta-analysis. Am J Clin Nutr 95(6):1357–1364. https://doi.org/10.3945/ajcn.111.031070
Urbain P, Singler F, Ihorst G, Biesalski H-K, Bertz H (2011) Bioavailability of vitamin D2 from UV-B-irradiated button mushrooms in healthy adults deficient in serum 25-hydroxyvitamin D: a randomized controlled trial. Eur J Clin Nutr 65(8):965–971. https://doi.org/10.1038/ejcn.2011.53
Wang Z, Lin YS, Zheng XE, Senn T, Hashizume T, Scian M et al (2012) An inducible cytochrome P450 3A4-dependent vitamin D catabolic pathway. Mol Pharmacol 81(4):498–509. https://doi.org/10.1124/mol.111.076356
Zhu JG, Ochalek JT, Kaufmann M, Jones G, Deluca HF (2013) CYP2R1 is a major, but not exclusive, contributor to 25-hydroxyvitamin D production in vivo. Proc Natl Acad Sci USA 110(39):S. 15650-15655. https://doi.org/10.1073/pnas.1315006110
Acknowledgements
Special thanks to Mark Genther for proof-reading the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
ACB: conceptualization, methodology, validation, formal analysis, investigation, writing—original draft. SP: validation, investigation; SS: validation, investigation; TW: formal analysis; MK: investigation, resources, writing—review & editing; SL: resources, supervision, writing—review & editing; GIS: conceptualization, resources, writing—review & editing, supervision; CD: conceptualization, investigation, resources, writing—review & editing, supervision, project administration.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no relevant conflict of interest.
Ethics approval
The study design was approved by the Ethics Committee of the Friedrich Schiller University Jena (No. 5417–01/18) and registered at clinicaltrials.gov (NCT03499327).
Consent to participate
Written consent was obtained from all participants before enrollment in this study.
Consent for publication
Not applicable.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bailer, A.C., Philipp, S., Staudt, S. et al. UVB-exposed wheat germ oil increases serum 25-hydroxyvitamin D2 without improving overall vitamin D status: a randomized controlled trial. Eur J Nutr 61, 2571–2583 (2022). https://doi.org/10.1007/s00394-022-02827-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-022-02827-w