Abstract
Purpose
Soluble fibre beneficially affects metabolism but whether it can augment the reductions in glycemia induced through intensive weight management has not been extensively studied. Our objective was to examine the adjunct effect of the soluble viscous fibre PGX® on glycemic control in adults with type 2 diabetes (T2D) enrolled in a year-long medically supervised weight management program.
Methods
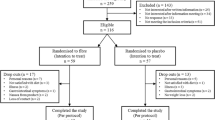
In a placebo-controlled, double-blind study, 290 adults with overweight/obesity and T2D were randomized to receive PGX (15–20 g/day) or isocaloric placebo (rice flour, 6.4–8.6 g/day) as an adjunct to intensive weight management for 52 weeks. The primary outcome was change in glycemic control (HbA1c). Other outcome measures included weight loss, blood lipids, blood pressure, cytokines and fecal microbiota.
Results
Compared to baseline HbA1c in PGX (7.2 ± 1.1%) and placebo (7.0 ± 0.9%) groups, there was a significant reduction at 16 and 26 weeks, however, only PGX showed a significant absolute reduction of 0.23% at 52 weeks; there were no between-group differences in HbA1c. At 52 weeks, only PGX significantly decreased body weight compared to baseline and reduced waist circumference at all time points. Compared to baseline, only PGX showed a significant reduction in LDL cholesterol at 16 and 26 weeks. PGX significantly increased the relative abundance of Collinsella, Parabacteroides and Roseburia.
Conclusion
Adding PGX to a weight management program for individuals with T2D provides a sustained reduction in HbA1c compared to placebo. Improvements in other metabolic outcomes suggest that PGX may be a promising adjunct to weight loss programs in patients with T2D.
Clinical trial
This trial was registered at ClinicalTrials.gov as NCT01644201.




Similar content being viewed by others
References
Chatterjee S, Khunti K, Davies MJ (2017) Type 2 diabetes. Lancet 389:3–9
Look AHEAD Research Group, Wing RR, Bolin P et al (2013) Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med 369:145–154
Dutton GR, Lewis CE (2015) The Look AHEAD trial: implications for lifestyle intervention in type 2 diabetes mellitus. Prog Cardiovasc Dis 58:69–75
Jenkins DJ, Jenkins AL (1985) Dietary fiber and the glycemic response. Proc Soc Exp Biol Med 180:422–431
Silva FM, Kramer CK, de Almeida JC, Steemburgo T, Luiz Gross J, Azevedo MJ (2013) Fiber intake and glycemic control in patients with type 2 diabetes mellitus: a systematic review with meta-analysis of randomized controlled trials. Nutr Rev 7:790–801
Jovanovski E, Khayyat R, Zurbau A, Komishon A, Mazhar N, Sievenpiper JL, Blanco Mejia S, Ho HVT, Li D, Jenkins AL, Duvnjak L, Vuksan V (2019) Should Viscous fiber supplements be considered in diabetes control? results from a systematic review and meta-analysis of randomized controlled trials. Diabetes Care 42:755–766
Jenkins AL, Kacinik V, Lyon M, Wolever TM (2010) Effect of adding the novel fiber, PGX®, to commonly consumed foods on glycemic response, glycemic index and GRIP: a simple and effective strategy for reducing post prandial blood glucose levels–a randomized, controlled trial. Nutr J 22(9):58
Brand-Miller JC, Atkinson FS, Gahler RJ, Kacinik V, Lyon MR, Wood S (2012) Effects of added PGX®, a novel functional fibre, on the glycaemic index of starchy foods. Br J Nutr 108:245–248
Reimer RA, Yamaguchi H, Eller LK, Lyon MR, Gahler RJ, Kacinik V, Juneja P, Wood S (2013) Changes in visceral adiposity and serum cholesterol with a novel viscous polysaccharide in Japanese adults with abdominal obesity. Obesity 21:E379–E387
Grover GJ, Koetzner L, Wicks J, Gahler R, Lyon MR, Reimer RA, Wood S (2011) Effects of the soluble fiber complex PolyGlycopleX on glucose homeostasis and body weight in young zucker diabetic rats. Front Pharmacol 2(47):1
Reimer RA, Grover GJ, Koetzner L, Gahler RJ, Lyon MR, Wood S (2014) Combining sitagliptin/metformin with a functional fiber delays diabetes progression in Zucker rats. J Endocrinol 220:361–373
Aviles-Santa L, Sinding J, Raskin P (1999) Effects of metformin in patients with poorly controlled, insulin-treated type 2 diabetes mellitus. A randomized, double-blind, placebo-controlled trial. Ann Intern Med 131:182–188
Wharton S, VanderLelie S, Sharma AM, Sharma S, Kuk JL (2012) Feasibility of an interdisciplinary program for obesity management in Canada. Can Fam Physician 58:e32–e38
Delahanty LM, Nathan DM (2008) Implications of the diabetes prevention program (DPP) and look AHEAD clinical trials for lifestyle interventions. J Am Diet Assoc 108(4 Suppl 1):S66–S72
Look AHEAD Research Group (2003) Look AHEAD (Action for Health in Diabetes): Design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Con Clin Trials 24:610–628
Pols MA, Peeters PH, Bueno-De-Mesquita HB, Ocke MC, Wentink CA, Kemper HC, Collette HJ (1995) Validity and repeatability of a modified Baecke questionnaire on physical activity. Int J Epidemiol 24:381–388
Bomhof MR, Saha DC, Reid DT, Paul HA, Reimer RA (2014) Combined effects of oligofructose and Bifidobacterium animalis on gut microbiota and glycemia in obese rats. Obesity 22:763–771
Bomhof MR, Paul HA, Geuking MB, Eller LK, Reimer RA (2016) Improvement in adiposity with oligofructose is modified by antibiotics in obese rats. FASEB J 30:2720–2732
Cole JR, Wang Q, Fish JA, Chai B, McGarrell DM, Sun Y, Brown CT, Porras-Alfaro A, Kuske CR, Tiedje JM (2014) Ribosomal Database Project: data and tools for high throughput rRNA analysis. Nucl Acids Res 42:633–642
Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C (2011) Metagenomic biomarker discovery and explanation. Genome Biol 12:R60
Hoaglin DC, Iglewicz B (1987) Fine tuning some resistant rules for outlier labeling, Journal of American Statistical Association. J Am Stat Assoc 82:1147–1149
Durrer Schutz D, Busetto L, Dicker D, Farpour-Lambert N, Pryke R, Toplak H, Widmer D, Yumuk V, Schutz Y (2019) European practical and patient-centred guidelines for adult obesity management in primary care. Obesity Facts 12:40–66
Diabetes Prevention Program Research Group (2009) 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 374:1677–1686
Wing RR, Lang W, Wadden TA, Safford M, Knowler WC, Bertoni AG, Hill JO, Brancati FL, Peters A, Wagenknecht L, Look AHEAD Research Group (2011) Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care 34:1481–1486
Holman RR, Cull CA, Turner RC (1999) A randomized double-blind trial of acarbose in type 2 diabetes shows improved glycemic control over 3 years (U.K. Prospective Diabetes Study 44). Diabetes Care 22:960–964
Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR (2000) Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. Br Med J 321:405–412
Tajfard M, Latiff LA, Rahimi HR, Moohebati M, Hasanzadeh M, Emrani AS, Esmaeily H, Taghipour A, Mirhafez SR, Ferns GA, Mardan-Nik M, Mohammadzadeh E, Avan A, Ghayour-Mobarhan M (2017) Serum concentrations of MCP-1 and IL-6 in combination predict the presence of coronary artery disease and mortality in subjects undergoing coronary angiography. Mol Cell Biochem 435:37–45
Knowler WC, Barrett-Connor E, Fowler SE et al (2002) Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346:393–403
Ohta A, Kato H, Ishii S, Sasaki Y, Nakamura Y, Nakagawa T, Nagai Y, Tanaka Y (2017) Ipragliflozin, a sodium glucose co-transporter 2 inhibitor, reduces intrahepatic lipid content and abdominal visceral fat volume in patients with type 2 diabetes. Expert Opin Pharmacother 18:1433–1438
Kashiwagi R, Iwahashi H, Yamada Y, Sakaue T, Okita T, Kawachi Y, Iwamoto R, Saisho K, Tamba S, Yamamoto K, Watanabe T, Fujimoto T, Matsuzawa Y (2017) Effective waist circumference reduction rate necessary to avoid the development of type 2 diabetes in Japanese men with abdominal obesity. Endocr J 64:881–894
Onakpoya IJ, Heneghan CJ (2015) Effect of the novel functionalfibre, polyglycoplex (PGX), on bodyweight and metabolic parameters: A systematic review of randomizedclinical trials. Clin Nutr 34:1109–1114
Reimer RA, Grover GJ, Koetzner L, Gahler R, Juneja P, Lyon MR, Wood S (2012) Sitagliptin reduces hyperglycemia and increases satiety hormone secretion more effectively when used with a novel polysaccharide in obese Zucker rats. J Nutr 142:1812–1820
Jane M, McKay J, Pal S (2019) Effects of daily consumption of psyllium, oat bran and polyGlycopleXon obesity-related disease risk factors: A critical review. Nutrition 57:84–91
Reimer RA, Maathuis AJH, Venema K, Lyon MR, Gahler RJ, Wood S (2014) Effect of the novel polysaccharide polyglycoplex® on short-chain fatty acid production in a computer-controlled in vitro model of the human large intestine. Nutrients 6:1115–1127
Hosseini E, Grootaert C, Verstraete W, Van de Wiele T (2011) Propionate as a health-promoting microbial metabolite in the human gut. Nutr Rev 69:245–258
Weitkunat K, Schumann S, Nickel D, Kappo KA, Petzke KJ, Kipp AP, Blaut M, Klaus S (2016) Importance of propionate for the repression of hepaticlipogenesis and improvement of insulin sensitivityin high-fat diet-induced obesity. Mol Nutr Food Res 60:2611–2621
Zhao Y, Liu J, Hao W, Zhu H, Liang N, He Z, Ma KY, Chen Z-Y (2017) Structure-specific effects of short-chain fatty acids on plasma cholesterol concentration in male syrian hamsters. J Agric Food Chem 65:10984–10992
Zhao L, Zhang F, Ding X, Wu G, Lam YY, Wang X, Fu H, Xue X, Lu C, Ma J, Yu L, Xu C, Ren Z, Xu Y, Xu S, Shen H, Zhu X, Shi Y, Shen Q, Dong W, Liu R, Ling Y, Zeng Y, Wang X, Zhang Q, Wang J, Wang L, Wu Y, Zeng B, Wei H, Zhang M, Peng Y, Zhang C (2018) Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 359:1151–1156
Lordan C, Thapa D, Ross RP, Cotter PD (2020) Potential for enriching next-generation health-promoting gut bacteria through prebiotics and other dietary components. Gut Microbes 11:1–20
Hiippala K, Jouhten H, Ronkainen A, Hartikainen A, Kainulainen V, Jalanka J, Satokari R (2018) The Potential of Gut Commensals in Reinforcing Intestinal Barrier Function and Alleviating Inflammation. Nutrients 10(8):988
Xu J, Lian F, Zhao L, Zhao Y, Chen X, Zhang X, Guo Y, Zhang C, Zhou Q, Xue Z, Pang X, Zhao L, Tong X (2015) Structural modulation of gut microbiota during alleviation of type 2 diabetes with a Chinese herbal formula. ISME J 9:552–562
Nicolucci AC, Hume MP, Martinez I, Mayengbam S, Walter J, Reimer RA (2017) Prebiotic reduces body fat and alters intestinal microbiota in children with overweight or obesity. Gastroenterology 153:711–722
Carlson JL, Erickson JM, Hess JM, Gould TJ, Slavin JL (2017) Prebiotic dietary fiber and gut health: comparing the in vitro fermentations of beta-glucan. Inulin and Xylooligosaccharide Nutrients 9(12):1–17
Dewulf EM, Cani PD, Claus SP, Fuentes S, Puylaert PG, Neyrinck AM, Bindels LB, de Vos WM, Gibson GR, Thissen JP, Delzenne NM (2013) Insight into the prebiotic concept: lessons from an exploratory, double blind intervention study with inulin-type fructans in obese women. Gut 62:1112–1121
Jiandani D, Wharton S, Rotondi MA, Ardern CI, Kuk JL (2016) Predictors of early attrition and successful weight loss in patients attending an obesity management program. BMC Obes Mar 9;3:14
EFSA Panel on Dietetics Products NaA, Turck D, Bresson JL et al (2017) Safety of alginate-konjac-xanthan polysaccharide complex (PGX) as a novel food pursuant to Regulation (EC) No 258/97. EFSA Journal Adopted 4 April 2017
Matulka RA, Lyon MR, Wood S, Marone PA, Merkel DJ, Burdock GA (2009) The safety of PolyGlycopleX® (PGX®) as shown in a 90-day rodent feeding study. Nutr J 8:1–11
Acknowledgements
This study was sponsored by InovoBiologic Inc., Calgary, Canada. RAR, SWharton, TG, PM, MRL, RJG and SWood designed the research. SWharton conducted the study. HRR performed microbiota bioinformatics. RAR analyzed data, wrote the paper and had primary responsibility for final content. All authors critically read and approved the final manuscript.
Funding
This study was sponsored by InovoBiologic Inc., Calgary, Canada.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
RAR and S Wood receive consulting fees from InovoBiologic Inc. RAR received financial support for the preparation of this manuscript. S Wharton received funds to pay clinical staff for the conduct of the study. S Wood has an InovoBiologic Inc. patent pending. MRL receives consulting fees from the Factors Group of Companies and holds various patents on PGX®. RJG is the owner of the Factors Group of Companies and holds various patents on PGX. TG, PM and HRR declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Reimer, R.A., Wharton, S., Green, T.J. et al. Effect of a functional fibre supplement on glycemic control when added to a year-long medically supervised weight management program in adults with type 2 diabetes. Eur J Nutr 60, 1237–1251 (2021). https://doi.org/10.1007/s00394-020-02328-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-020-02328-8