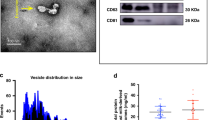
Abstract
Purpose
Bovine milk exosomes, which are enriched with microRNAs (miRNAs) and proteins, regulate immune response and growth. In the present study, we aimed to assess the protective effects of bovine milk exosomes against oxidative stress of intestinal crypt epithelial cells (IEC-6).
Methods
Bovine milk exosomes were isolated and characterized. To assess the protective effects of exosomes, IEC-6 cells were pretreated with exosomes, followed by H2O2. Cell viability and levels of superoxide dismutase (SOD), malondialdehyde (MDA), glutathione peroxidase (GPX), reactive oxidative species (ROS), and lactate dehydrogenase (LDH) were measured. The expression levels of nuclear factor erythroid 2-related factor 2 (Nrf2) and heme oxygenase 1 (Ho1) genes, and miR-146a, miR-155, and the HO-1 protein were also determined.
Results
The isolated bovine milk exosome were positive for CD63 and CD9 expression. The exosomes were approximately circular and had a diameter of about 67.23 nm. Pretreatment of IEC-6 cells with bovine milk exosomes enhanced cell viability; increased SOD and GPX activities; and reduced LDH, ROS, and MDA levels after H2O2 challenge. Further analysis showed that exosome pretreatment increased intracellular miR-146a and miR-155 levels. Exosome pretreatment inhibited the elevation of Nrf2 and Ho1 gene expression induced by H2O2, but promoted HO-1 protein expression.
Conclusion
The results indicated that bovine milk exosomes exerted protective effects against oxidative stress in IEC-6 cells.






Similar content being viewed by others
References
Bhattacharyya A, Chattopadhyay R, Mitra S, Crowe SE (2014) Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol Rev 94(2):329–354. https://doi.org/10.1152/physrev.00040.2012
Aviello G, Knaus UG (2017) ROS in gastrointestinal inflammation: Rescue Or Sabotage? Br J Pharmacol 174(12):1704–1718. https://doi.org/10.1111/bph.13428
Peng YC, Hsu CL, Tung CF, Chou WK, Huang LR, Hung DZ, Hu WH, Yang DY (2008) Chemiluminescence assay of mucosal reactive oxygen species in gastric cancer, ulcer and antral mucosa. Hepatogastroenterology 55(82–83):770–773
Grisham MB (1994) Oxidants and free radicals in inflammatory bowel disease. Lancet 344(8926):859–861
Pavlick KP, Laroux FS, Fuseler J, Wolf RE, Gray L, Hoffman J, Grisham MB (2002) Role of reactive metabolites of oxygen and nitrogen in inflammatory bowel disease. Free Radic Biol Med 33(3):311–322
Tian T, Wang Z, Zhang J (2017) Pathomechanisms of oxidative stress in inflammatory bowel disease and potential antioxidant therapies. Oxid Med Cell Longev 2017:4535194. https://doi.org/10.1155/2017/4535194
Kekec Y, Paydas S, Tuli A, Zorludemir S, Sakman G, Seydaoglu G (2009) Antioxidant enzyme levels in cases with gastrointesinal cancer. Eur J Intern Med 20(4):403–406. https://doi.org/10.1016/j.ejim.2008.12.003
Inokuma T, Haraguchi M, Fujita F, Tajima Y, Kanematsu T (2009) Oxidative stress and tumor progression in colorectal cancer. Hepatogastroenterology 56(90):343–347
Haug A, Hostmark AT, Harstad OM (2007) Bovine milk in human nutrition—a review. Lipids Health Dis 6:25. https://doi.org/10.1186/1476-511x-6-25
Arntz OJ, Pieters BC, Oliveira MC, Broeren MG, Bennink MB, de Vries M, van Lent PL, Koenders MI, van den Berg WB, van der Kraan PM, van de Loo FA (2015) Oral administration of bovine milk derived extracellular vesicles attenuates arthritis in two mouse models. Mol Nutr Food Res 59(9):1701–1712. https://doi.org/10.1002/mnfr.201500222
Mansson HL (2008) Fatty acids in bovine milk fat. Food Nutr Res https://doi.org/10.3402/fnr.v52i0.1821
Admyre C, Johansson SM, Qazi KR, Filen JJ, Lahesmaa R, Norman M, Neve EP, Scheynius A, Gabrielsson S (2007) Exosomes with immune modulatory features are present in human breast milk. J Immunol 179(3):1969–1978
Hata T, Murakami K, Nakatani H, Yamamoto Y, Matsuda T, Aoki N (2010) Isolation of bovine milk-derived microvesicles carrying mRNAs and microRNAs. Biochem Biophys Res Commun 396(2):528–533. https://doi.org/10.1016/j.bbrc.2010.04.135
Thery C, Zitvogel L, Amigorena S (2002) Exosomes: composition, biogenesis and function. Nat Rev Immunol 2(8):569–579. https://doi.org/10.1038/nri855
Raposo G, Stoorvogel W (2013) Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol 200(4):373–383. https://doi.org/10.1083/jcb.201211138
Huang X, Yuan T, Tschannen M, Sun Z, Jacob H, Du M, Liang M, Dittmar RL, Liu Y, Liang M, Kohli M, Thibodeau SN, Boardman L, Wang L (2013) Characterization of human plasma-derived exosomal RNAs by deep sequencing. BMC Genom 14:319. https://doi.org/10.1186/1471-2164-14-319
Izumi H, Tsuda M, Sato Y, Kosaka N, Ochiya T, Iwamoto H, Namba K, Takeda Y (2015) Bovine milk exosomes contain microRNA and mRNA and are taken up by human macrophages. J Dairy Sci 98(5):2920–2933. https://doi.org/10.3168/jds.2014-9076
Abels ER, Breakefield XO (2016) Introduction to extracellular vesicles: biogenesis, rna cargo selection, content, release, and uptake. Cell Mol Neurobiol 36(3):301–312. https://doi.org/10.1007/s10571-016-0366-z
Freedman JE, Gerstein M, Mick E, Rozowsky J, Levy D, Kitchen R, Das S, Shah R, Danielson K, Beaulieu L, Navarro FC, Wang Y, Galeev TR, Holman A, Kwong RY, Murthy V, Tanriverdi SE, Koupenova-Zamor M, Mikhalev E, Tanriverdi K (2016) Diverse human extracellular RNAs are widely detected in human plasma. Nat Commun 7:11106. https://doi.org/10.1038/ncomms11106
Greening DW, Gopal SK, Xu R, Simpson RJ, Chen W (2015) Exosomes and their roles in immune regulation and cancer. Semin Cell Dev Biol 40:72–81. https://doi.org/10.1016/j.semcdb.2015.02.009
Gangoda L, Boukouris S, Liem M, Kalra H, Mathivanan S (2015) Extracellular vesicles including exosomes are mediators of signal transduction: are they protective or pathogenic? Proteomics 15(2–3):260–271. https://doi.org/10.1002/pmic.201400234
Mittelbrunn M, Gutierrez-Vazquez C, Villarroya-Beltri C, Gonzalez S, Sanchez-Cabo F, Gonzalez MA, Bernad A, Sanchez-Madrid F (2011) Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun 2:282. https://doi.org/10.1038/ncomms1285
Yanez-Mo M, Siljander PR, Andreu Z, Zavec AB, Borras FE, Buzas EI, Buzas K, Casal E, Cappello F, Carvalho J, Colas E, Cordeiro-da Silva A, Fais S, Falcon-Perez JM, Ghobrial IM, Giebel B, Gimona M, Graner M, Gursel I, Gursel M, Heegaard NH, Hendrix A, Kierulf P, Kokubun K, Kosanovic M, Kralj-Iglic V, Kramer-Albers EM, Laitinen S, Lasser C, Lener T, Ligeti E, Line A, Lipps G, Llorente A, Lotvall J, Mancek-Keber M, Marcilla A, Mittelbrunn M, Nazarenko I, Nolte-'t Hoen EN, Nyman TA, O'Driscoll L, Olivan M, Oliveira C, Pallinger E, Del Portillo HA, Reventos J, Rigau M, Rohde E, Sammar M, Sanchez-Madrid F, Santarem N, Schallmoser K, Ostenfeld MS, Stoorvogel W, Stukelj R, Van der Grein SG, Vasconcelos MH, Wauben MH, De Wever O (2015) Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles 4:27066. https://doi.org/10.3402/jev.v4.27066
Yamashita T, Takahashi Y, Takakura Y (2018) Possibility of exosome-based therapeutics and challenges in production of exosomes eligible for therapeutic application. Biol Pharm Bull 41(6):835–842. https://doi.org/10.1248/bpb.b18-00133
Melnik BC, John SM, Schmitz G (2014) Milk: an exosomal microRNA transmitter promoting thymic regulatory T cell maturation preventing the development of atopy? J Transl Med 12:43. https://doi.org/10.1186/1479-5876-12-43
Nordgren TM, Heires AJ, Zempleni J, Swanson BJ, Wichman C, Romberger DJ (2019) Bovine milk-derived extracellular vesicles enhance inflammation and promote M1 polarization following agricultural dust exposure in mice. J Nutr Biochem 64:110–120. https://doi.org/10.1016/j.jnutbio.2018.10.017
Munir J, Lee M, Ryu S (2019) Exosomes in food: health benefits and clinical relevance in diseases. Adv Nutr. https://doi.org/10.1093/advances/nmz123
Zhou F, Paz HA, Sadri M, Cui J, Kachman SD, Fernando SC, Zempleni J (2019) Dietary bovine milk exosomes elicit changes in bacterial communities in C57BL/6 mice. Am J Physiol Gastrointest Liver Physiol 317(5):G618–G624. https://doi.org/10.1152/ajpgi.00160.2019
Li B, Hock A, Wu RY, Minich A, Botts SR, Lee C, Antounians L, Miyake H, Koike Y, Chen Y, Zani A, Sherman PM, Pierro A (2019) Bovine milk-derived exosomes enhance goblet cell activity and prevent the development of experimental necrotizing enterocolitis. PLoS ONE 14(1):e0211431. https://doi.org/10.1371/journal.pone.0211431
Martin C, Patel M, Williams S, Arora H, Sims B (2018) Human breast milk-derived exosomes attenuate cell death in intestinal epithelial cells. Innate Immun 24(5):278–284. https://doi.org/10.1177/1753425918785715
Chen T, Xie MY, Sun JJ, Ye RS, Cheng X, Sun RP, Wei LM, Li M, Lin DL, Jiang QY, Xi QY, Zhang YL (2016) Porcine milk-derived exosomes promote proliferation of intestinal epithelial cells. Sci Rep 6:33862. https://doi.org/10.1038/srep33862
Yu S, Zhao Z, Sun L, Li P (2017) Fermentation results in quantitative changes in milk-derived exosomes and different effects on cell growth and survival. J Agric Food Chem 65(6):1220–1228. https://doi.org/10.1021/acs.jafc.6b05002
Leiferman A, Shu J, Grove R, Cui J, Adamec J, Zempleni J (2018) A diet defined by its content of bovine milk exosomes and their RNA cargos has moderate effects on gene expression, amino acid profiles and grip strength in skeletal muscle in C57BL/6 mice. J Nutr Biochem 59:123–128. https://doi.org/10.1016/j.jnutbio.2018.06.007
Mutai E, Zhou F, Zempleni J (2017) Depletion of dietary bovine milk exosomes impairs sensorimotor gating and spatial learning in C57BL/6 Mice. FASEB J 31(1_supplement):150.154–150.154. https://doi.org/10.1096/fasebj.31.1_supplement.150.4
Aguilar-Lozano A, Baier S, Grove R, Shu J, Giraud D, Leiferman A, Mercer KE, Cui J, Badger TM, Adamec J, Andres A, Zempleni J (2018) Concentrations of purine metabolites are elevated in fluids from adults and infants and in livers from mice fed diets depleted of bovine milk exosomes and their RNA cargos. J Nutr 148(12):1886–1894. https://doi.org/10.1093/jn/nxy223
Sadri M, Xie F, Wood J, Zempleni J (2016) Dietary depletion of cow’s Milk microRNAs impairs fecundity in mice. FASEB J 30(1_supplement):673.675–673.675. https://doi.org/10.1096/fasebj.30.1_supplement.673.5
Zempleni J, Sukreet S, Zhou F, Wu D, Mutai E (2019) Milk-Derived Exosomes and metabolic regulation. Annu Rev Anim Biosci 7:245–262. https://doi.org/10.1146/annurev-animal-020518-115300
Kusuma RJ, Manca S, Friemel T, Sukreet S, Nguyen C, Zempleni J (2016) Human vascular endothelial cells transport foreign exosomes from cow's milk by endocytosis. Am J Physiol Cell Physiol:ajpcell 00169:02015. https://doi.org/10.1152/ajpcell.00169.2015
Jo HS, Kim DS, Ahn EH, Kim DW, Shin MJ, Cho SB, Park JH, Lee CH, Yeo EJ, Choi YJ, Yeo HJ, Chung CS, Cho SW, Han KH, Park J, Eum WS, Choi SY (2016) Protective effects of Tat-NQO1 against oxidative stress-induced HT-22 cell damage, and ischemic injury in animals. BMB Rep 49(11):617–622
Zhang J, Cai S, Li J, Xiong L, Tian L, Liu J, Huang J, Liu Z (2016) Neuroprotective effects of theaflavins against oxidative stress-induced apoptosis in PC12 cells. Neurochem Res 41(12):3364–3372. https://doi.org/10.1007/s11064-016-2069-8
Bhatti FU, Mehmood A, Latief N, Zahra S, Cho H, Khan SN, Riazuddin S (2017) Vitamin E protects rat mesenchymal stem cells against hydrogen peroxide-induced oxidative stress in vitro and improves their therapeutic potential in surgically-induced rat model of osteoarthritis. Osteoarthr Cartil 25(2):321–331. https://doi.org/10.1016/j.joca.2016.09.014
Bettaib J, Talarmin H, Kalai FZ, Giroux-Metges MA, Ksouri R (2017) Limoniastrum guyonianum prevents H2O2-induced oxidative damage in IEC-6 cells by enhancing enzyamtic defense, reducing glutathione depletion and JNK phosphorylation. Biomed Pharmacother 95:1404–1411. https://doi.org/10.1016/j.biopha.2017.09.068
Zou L, Sato N, Kone BC (2004) Alpha-melanocyte stimulating hormone protects against H2O2-induced inhibition of wound restitution in IEC-6 cells via a Syk kinase- and NF-kappabeta-dependent mechanism. Shock 22(5):453–459
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Zhou Y, Xu H, Xu W, Wang B, Wu H, Tao Y, Zhang B, Wang M, Mao F, Yan Y, Gao S, Gu H, Zhu W, Qian H (2013) Exosomes released by human umbilical cord mesenchymal stem cells protect against cisplatin-induced renal oxidative stress and apoptosis in vivo and in vitro. Stem Cell Res Ther 4(2):34. https://doi.org/10.1186/scrt194
Tan CY, Lai RC, Wong W, Dan YY, Lim SK, Ho HK (2014) Mesenchymal stem cell-derived exosomes promote hepatic regeneration in drug-induced liver injury models. Stem Cell Res Ther 5(3):76. https://doi.org/10.1186/scrt465
de Godoy MA, Saraiva LM, de Carvalho LRP, Vasconcelos-Dos-Santos A, Beiral HJV, Ramos AB, Silva LRP, Leal RB, Monteiro VHS, Braga CV, de Araujo-Silva CA, Sinis LC, Bodart-Santos V, Kasai-Brunswick TH, Alcantara CL, Lima A, da Cunha ESNL, Galina A, Vieyra A, De Felice FG, Mendez-Otero R, Ferreira ST (2018) Mesenchymal stem cells and cell-derived extracellular vesicles protect hippocampal neurons from oxidative stress and synapse damage induced by amyloid-beta oligomers. J Biol Chem 293(6):1957–1975. https://doi.org/10.1074/jbc.M117.807180
Arslan F, Lai RC, Smeets MB, Akeroyd L, Choo A, Aguor EN, Timmers L, van Rijen HV, Doevendans PA, Pasterkamp G, Lim SK, de Kleijn DP (2013) Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res 10(3):301–312. https://doi.org/10.1016/j.scr.2013.01.002
Badawy AA, El-Magd MA, AlSadrah SA (2018) Therapeutic effect of camel milk and its exosomes on MCF7 cells in vitro and in vivo. Integr Cancer Ther 17(4):1235–1246. https://doi.org/10.1177/1534735418786000
Shoji H, Oguchi S, Shinohara K, Shimizu T, Yamashiro Y (2007) Effects of iron-unsaturated human lactoferrin on hydrogen peroxide-induced oxidative damage in intestinal epithelial cells. Pediatr Res 61(1):89–92. https://doi.org/10.1203/01.pdr.0000250198.22735.20
Kensler TW, Wakabayashi N, Biswal S (2007) Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol 47:89–116. https://doi.org/10.1146/annurev.pharmtox.46.120604.141046
Sun W, Julie Li YS, Huang HD, Shyy JY, Chien S (2010) microRNA: a master regulator of cellular processes for bioengineering systems. Annu Rev Biomed Eng 12:1–27. https://doi.org/10.1146/annurev-bioeng-070909-105314
Catalanotto C, Cogoni C, Zardo G (2016) MicroRNA in control of gene expression: an overview of nuclear functions. Int J Mol Sci. https://doi.org/10.3390/ijms17101712
Vidigal JA, Ventura A (2015) The biological functions of miRNAs: lessons from in vivo studies. Trends Cell Biol 25(3):137–147. https://doi.org/10.1016/j.tcb.2014.11.004
Bartel DP (2009) MicroRNAs: target recognition and regulatory functions. Cell 136(2):215–233. https://doi.org/10.1016/j.cell.2009.01.002
Wan G, Liu Y, Han C, Zhang X, Lu X (2014) Noncoding RNAs in DNA repair and genome integrity. Antioxid Redox Signal 20(4):655–677. https://doi.org/10.1089/ars.2013.5514
Bu H, Wedel S, Cavinato M, Jansen-Durr P (2017) MicroRNA regulation of oxidative stress-induced cellular senescence. Oxid Med Cell Longev 2017:2398696. https://doi.org/10.1155/2017/2398696
Espinosa-Diez C, Miguel V, Mennerich D, Kietzmann T, Sanchez-Perez P, Cadenas S, Lamas S (2015) Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol 6:183–197. https://doi.org/10.1016/j.redox.2015.07.008
Paladino S, Conte A, Caggiano R, Pierantoni GM, Faraonio R (2018) Nrf2 pathway in age-related neurological disorders: insights into MicroRNAs. Cell Physiol Biochem 47(5):1951–1976. https://doi.org/10.1159/000491465
Xie Y, Chen Y (2016) microRNAs: emerging targets regulating oxidative stress in the models of Parkinson's disease. Front Neurosci 10:298. https://doi.org/10.3389/fnins.2016.00298
Matouskova P, Hanouskova B, Skalova L (2018) MicroRNAs as potential regulators of glutathione peroxidases expression and their role in obesity and related pathologies. Int J Mol Sci. https://doi.org/10.3390/ijms19041199
Gong YY, Luo JY, Wang L, Huang Y (2018) MicroRNAs regulating reactive oxygen species in cardiovascular diseases. Antioxid Redox Signal 29(11):1092–1107. https://doi.org/10.1089/ars.2017.7328
Izumi H, Kosaka N, Shimizu T, Sekine K, Ochiya T, Takase M (2012) Bovine milk contains microRNA and messenger RNA that are stable under degradative conditions. J Dairy Sci 95(9):4831–4841. https://doi.org/10.3168/jds.2012-5489
Izumi H, Kosaka N, Shimizu T, Sekine K, Ochiya T, Takase M (2013) Purification of RNA from milk whey. Methods Mol Biol 1024:191–201. https://doi.org/10.1007/978-1-62703-453-1_15
Sun Q, Chen X, Yu J, Zen K, Zhang CY, Li L (2013) Immune modulatory function of abundant immune-related microRNAs in microvesicles from bovine colostrum. Protein Cell 4(3):197–210. https://doi.org/10.1007/s13238-013-2119-9
Chen C, Jiang X, Gu S, Zhang Z (2017) MicroRNA-155 regulates arsenite-induced malignant transformation by targeting Nrf2-mediated oxidative damage in human bronchial epithelial cells. Toxicol Lett 278:38–47. https://doi.org/10.1016/j.toxlet.2017.07.215
Cheng X, Ku CH, Siow RC (2013) Regulation of the Nrf2 antioxidant pathway by microRNAs: new players in micromanaging redox homeostasis. Free Radic Biol Med 64:4–11. https://doi.org/10.1016/j.freeradbiomed.2013.07.025
Smith EJ, Shay KP, Thomas NO, Butler JA, Finlay LF, Hagen TM (2015) Age-related loss of hepatic Nrf2 protein homeostasis: Potential role for heightened expression of miR-146a. Free Radic Biol Med 89:1184–1191. https://doi.org/10.1016/j.freeradbiomed.2015.11.003
Tebay LE, Robertson H, Durant ST, Vitale SR, Penning TM, Dinkova-Kostova AT, Hayes JD (2015) Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative disease. Free Radic Biol Med 88(Pt B):108–146. https://doi.org/10.1016/j.freeradbiomed.2015.06.021
Yanaka A (2018) Role of NRF2 in protection of the gastrointestinal tract against oxidative stress. J Clin Biochem Nutr 63(1):18–25. https://doi.org/10.3164/jcbn.17-139
Ma Q (2013) Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol 53:401–426. https://doi.org/10.1146/annurev-pharmtox-011112-140320
Lee JM, Johnson JA (2004) An important role of Nrf2-ARE pathway in the cellular defense mechanism. J Biochem Mol Biol 37(2):139–143
Nguyen T, Nioi P, Pickett CB (2009) The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem 284(20):13291–13295. https://doi.org/10.1074/jbc.R900010200
Vanella L, Sanford C Jr, Kim DH, Abraham NG, Ebraheim N (2012) Oxidative stress and heme oxygenase-1 regulated human mesenchymal stem cells differentiation. Int J Hypertens 2012:890671. https://doi.org/10.1155/2012/890671
Wen Z, Liu W, Li X, Chen W, Liu Z, Wen J, Liu Z (2019) A Protective role of the NRF2-Keap1 pathway in maintaining intestinal barrier function. Oxid Med Cell Longev 2019:1759149. https://doi.org/10.1155/2019/1759149
Cheleschi S, De Palma A, Pascarelli NA, Giordano N, Galeazzi M, Tenti S, Fioravanti A (2017) Could oxidative stress regulate the expression of MicroRNA-146a and MicroRNA-34a in human osteoarthritic chondrocyte cultures? Int J Mol Sci. https://doi.org/10.3390/ijms18122660
Adesso S, Russo R, Quaroni A, Autore G, Marzocco S (2018) Astragalus membranaceus extract attenuates inflammation and oxidative stress in intestinal epithelial cells via NF-kappaB activation and Nrf2 response. Int J Mol Sci. https://doi.org/10.3390/ijms19030800
Zhuang S, Yu R, Zhong J, Liu P, Liu Z (2019) Rhein from rheum rhabarbarum inhibits hydrogen-peroxide-induced oxidative stress in intestinal epithelial cells partly through PI3K/Akt-mediated Nrf2/HO-1 pathways. J Agric Food Chem 67(9):2519–2529. https://doi.org/10.1021/acs.jafc.9b00037
Pulkkinen KH, Yla-Herttuala S, Levonen AL (2011) Heme oxygenase 1 is induced by miR-155 via reduced BACH1 translation in endothelial cells. Free Radic Biol Med 51(11):2124–2131. https://doi.org/10.1016/j.freeradbiomed.2011.09.014
Gu S, Lai Y, Chen H, Liu Y, Zhang Z (2017) miR-155 mediates arsenic trioxide resistance by activating Nrf2 and suppressing apoptosis in lung cancer cells. Sci Rep 7(1):12155. https://doi.org/10.1038/s41598-017-06061-x
Onodera Y, Teramura T, Takehara T, Obora K, Mori T, Fukuda K (2017) miR-155 induces ROS generation through downregulation of antioxidation-related genes in mesenchymal stem cells. Aging Cell 16(6):1369–1380. https://doi.org/10.1111/acel.12680
Sun J, Aswath K, Schroeder SG, Lippolis JD, Reinhardt TA, Sonstegard TS (2015) MicroRNA expression profiles of bovine milk exosomes in response to Staphylococcus aureus infection. BMC Genom 16(1):806. https://doi.org/10.1186/s12864-015-2044-9
Li R, Dudemaine PL, Zhao X, Lei C, Ibeagha-Awemu EM (2016) Comparative analysis of the miRNome of bovine milk fat, Whey and Cells. PLoS ONE 11(4):e0154129. https://doi.org/10.1371/journal.pone.0154129
Acknowledgements
This work was supported by the National Natural Science Foundation of China [Grant Number 21476176], the National Key Technology R&D Program of China [Grant Number 2015BAD16B01], the National High Technology Research and Development Program of China [863 Program, Grant Number 2015AA021002], and the International Science and Technology Cooperation Programs of Anhui [Grant Number 1503062006].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, L., Shi, Z., Wang, X. et al. Protective effects of bovine milk exosomes against oxidative stress in IEC-6 cells. Eur J Nutr 60, 317–327 (2021). https://doi.org/10.1007/s00394-020-02242-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-020-02242-z