Abstract
Purpose
To investigate the longitudinal association between the macronutrient composition of the diet and frailty.
Methods
Data were obtained from 5205 Dutch middle-aged and older adults participating in the Rotterdam Study. Frailty was measured using a frailty index based on the accumulation of 38 health-related deficits, score between 0 and 100, and a higher score indicating more frailty. Frailty was assessed at baseline and 11 years later (range of 23 years). Macronutrient intake was assessed using food-frequency questionnaires. The association between macronutrients and frailty over time was evaluated using multivariable linear regression, adjusted for the frailty index at baseline, energy intake, and other relevant confounders. All analyses were performed in strata of BMI.
Results
Median frailty index score was 13.8 points (IQR 9.6; 19.1) at baseline and increased by a median of 2.3 points (IQR − 2.0; 7.6) after 11 years. Overall, we found no significant associations between intake of carbohydrates or fat and frailty over time. We did observe a significant positive association between an iso-energetic intake of 10 g protein and frailty over time (β 0.31 (95% CI 0.06; 0.55)) which was mainly driven by animal protein (β 0.31 (95% CI 0.07; 0.56)). It did not depend on whether it was substituted fat or carbohydrates.
Conclusions
Our findings suggest that a reduction in the intake of animal protein may improve the overall health status over time in a relatively healthy population. More research is needed on the optimal macronutrient composition of the diet and frailty in more vulnerable populations.
Similar content being viewed by others
Introduction
The rapid aging of our population is a major public health issue [1]. A longer lifespan is often accompanied by an increased risk of disability and mortality, including the appearance of chronic diseases such as cardiovascular disorders, cancer, stroke, and dementia [2]. In addition to the focus on chronic diseases, a high amount of research tries to capture overall health. Overall health is determined by the accumulation of a wide range of health problems, including symptoms, signs, diseases, and disabilities [3], and not merely the absence of chronic diseases [4].
One way to assess overall health is via frailty, generally described as a non-specific state of homeostatic dysregulation in multiple systems, and vulnerability to stressors, such as illness, injury, or psychological stress [5, 6]. Frailty is a strong predictor for adverse events, including disability, institutionalization, hospitalization, and mortality [7, 8]. There are two well-known operationalizations of frailty: physical frailty and multidimensional frailty. Physical frailty, based on the presence of at least three of the following five criteria: weight loss, weak grip strength, exhaustion, slow gait speed, and low physical activity, mainly focused on predefined physical variables [5]. While multidimensional frailty covers a broad range of health domains, combining indicators on cognition, disabilities, biochemical abnormalities, and diseases [3, 9]. Indicators on separate health domains have only small effects on health; their cumulative effect becomes significant [10].
Multidimensional frailty focuses on a more holistic approach to treatment, rather single health deficits
For healthy aging, it is important to counteract the onset and progression of frailty. Different lifestyle factors play an important role in the prevention of frailty. One important modifiable factor is nutrition, by providing energy which is important for the overall homeostasis and by proving essential nutrients, necessary for the maintenance of bodily and organ functions [11]. So far, a recent literature review showed that most studies have focused on the association between protein and physical frailty [12]. High protein intake is shown to be beneficiary for physical frailty including muscle mass and muscle strength [13]. Nonetheless, far too little attention has been put to the association between macronutrients in general and more holistic approaches such as the frailty index. Considering multidimensional frailty, macronutrients intake might be beneficiary for some health domains but harmful for other health domains. For example, on one hand, a high protein diet is associated with higher satiety and lower total caloric intake, and lower body weight, and less body adiposity [14,15,16,17]. However, on the other hand, it is suggested that high protein intake might be harmful to kidney function [18]. Also, two systematic reviews concluded that high protein intake but a low carbohydrate intake was associated with higher all-cause mortality risk [19, 20]. Similar, a high carbohydrate or fat intake is associated with an increased coronary heart disease risk and a higher body mass index [21]; on the other hand, overweight might have a lower all-cause-mortality compared to normal weight at an older age [22].
To our knowledge, only a few studies investigated the association between diet and the frailty index, all focused on diet quality. These studies showed that better diet quality is associated with less frailty [23,24,25]. No studies are available on macronutrient intake and multidimensional frailty. We hypothesize that the macronutrient composition of the diet is of influence on the frailty index. The aim of the current study is to examine the longitudinal association of macronutrient intake with the frailty index, taking total energy intake and the overall macronutrient composition of the diet into account.
Methodology
Study design and participants
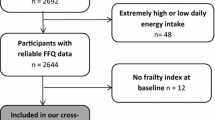
Data were obtained from the Rotterdam Study (RS), a population-based prospective cohort of middle-aged and older adults. The design of the Rotterdam Study has extensively been described elsewhere [26]. Briefly, the Rotterdam Study started in 1990, inviting all residents aged 55 years and over (n = 10,235) in a specific suburb of Rotterdam, from which 7983 took part in the RS’s first cohort (RS-I). The study was extended with new participants in 2000, inviting all residents aged 55 years and over or who moved into the study area (RS-II; n = 3011). In 2006, the study was extended with a third cohort, inviting all residents aged 45 years and over (RS-III; n = 3932). Data collection for all cohorts at baseline included questionnaires and an interview at home (2 h) by trained research assistants on among others activities of daily living, current health status, medical history, diet, medication use, smoking, highest obtained education, and physical activity. Additionally, participants visited our dedicated study center in the center of their district where physical examinations took place; stressing on body size, imaging, collection of body fluids, physical functioning, and cognitive performance. Examinations were repeated in each cohort every 3–5 years. For the current study, we excluded participants if their energy intake was implausible, having an estimated energy intake lower than 500 kcal or higher than 5000 kcal per day. Participants were included with a valid frailty index at baseline and follow-up, resulting in a total study population 5205 participants.
Dietary assessment
Dietary intake was assessed using validated Food-Frequency Questionnaires (FFQ), described in detail elsewhere [27]. Briefly, in RS-I-1 and RS-II-1, participants completed a checklist at home about foods and drinks which they consumed at least twice a month during the preceding year. Thereafter, trained dietitians interviewed the participants at the research center, using a validated, computerized 170-item semi-quantitative FFQ. This FFQ was previously validated against fifteen 24 h food records and four 24 h urinary urea excretion samples in a subsample of the RS, and showed good validity for macronutrient intakes (r for protein 0.61, r for fat 0.70, and r for carbohydrates 0.72) [28]. In RS-III-1, dietary intake was measured with a self-administrated, semi-quantitative FFQ. This FFQ was validated, in two other Dutch populations using a 9-day dietary record and a 4-week dietary history, and showed moderate-to-good validity for macronutrient intakes (r for protein 0.61, r for fat 0.47, and r for carbohydrates 0.71) [29]. This FFQ included 389 items on the frequency and amount of consumed food items over the last month. For the calculation of macronutrient intakes, the Dutch food composition database (NEVO) was used [30]. We calculated intake of the following macronutrients which were included in the analyses: total carbohydrates, mono- and disaccharides, polysaccharides, total fat, saturated fatty acids mono-unsaturated fatty acids, poly-unsaturated fatty acids, total protein, animal protein, and vegetable protein. Additionally, we calculated the intake of dietary fibers and alcohol intake in energy percentages, which were included as confounders in the analyses. Participants were excluded from the analyses.
Frailty index
Frailty was derived from the frailty index, previously designed for and validated in the Rotterdam Study [31]. The frailty index was assessed in RS-I-3, RS-I-5, RS-II-1, RS-II-2, RS-III-1, and RS-III-2 [26]. Of the original Rotterdam Frailty index (45 items), seven items (vitamin D, sex hormone binding globulin, mobility, uric acid, pro-brain natriuretic peptide, homocysteine, and C-reactive protein) were removed, because these items were not assessed at follow-up. De Haas et al. showed that the original Rotterdam Study frailty index and the adapted version of the frailty index (r = 0.98) had no major differences in frailty [25]. The frailty index consisted of 38 deficits, covering different health domains: functional status (n = 13), cognition (n = 6), diseases (n = 6), health conditions (n = 6), nutritional status (n = 3), and mood (n = 4). Deficits were dichotomized or categorized, based on previously predefined cut-off values [31] into a score ranging from 0 (deficit not present) till 1 (deficit present). Per person, the sum of all deficits was divided by the total number of deficits, resulting in a score ranging from 0 (no deficits present, least frail) till 1 (all deficits present, extremely frail). For the interpretation of the data, the frailty index score was multiplied by 100, resulting in a range from 0 to 100.
Other study parameters
Smoking status was classified as never, former, or current smoker. Level of education was determined by the highest attained education and recorded in four categories: low (primary education and lower vocational education), middle (secondary general education and secondary vocational education), middle–high (higher general education), and high (higher vocational education or university education). Net monthly household income was classified as low (< 1200€), middle (1200–2100€), and high (≥ 2100€). For RS-I and RS-II, physical activity was measured with an adapted version of the Zutphen Physical Activity Questionnaire [32], whereas for RS-III, the validated LASA physical activity Questionnaire (LAPAQ) [33] was used. Metabolic equivalents of task (MET) scores were calculated for the physical activities, weighted by their intensity, according to the compendium of physical activities 2011 [34]. Subsequently, MET hours per week were calculated for each participant. To take the differences of the questionnaires into account, MET hours per week were standardized by cohort (RS-I, RS-II, and RS-III) by calculating Z scores. Body height and weight were measured standing in light clothes, without shoes. BMI was calculated as weight divided by height squared (kg/m2) and defined as: normal weight (BMI ≤ 25 kg/m2), overweight (BMI 25–30 kg/m2), and obese (BMI > 30 kg/m2).
Statistical analysis
Baseline characteristics of the study population were provided as the median and interquartile range (IQR) and as frequency (percentage). Based on literature [22, 35] and a statistically significant interaction between carbohydrate, fat, and protein intake and BMI (P for interaction < 0.01), BMI was considered an effect modifier and all results are presented by the total population and by strata of BMI. Differences in baseline characteristics between strata of BMI were assessed by analysis of variance or Chi-square test.
The association between macronutrients and the frailty index was assessed using multivariable linear regression analyses. In all models, the frailty index at follow-up was included as the dependent variable and macronutrient intakes as the independent variables. Two methods to adjust for total energy intake were used. First, we applied the nutrient residual method and included the macronutrient intake adjusted for total energy intake, modeled as an increase of 10 g/day macronutrient [36]. Coefficients can be interpreted as the difference in frailty index score per increase of 10 g/day intake of a specific macronutrient keeping energy intake constant (iso-energetic) and as a result lower intake in one or more of the other macronutrients. Second, we applied the nutrient density method by including macronutrient separately (per five energy percentage) as well as summed to represent total energy intake. By excluding, for example, protein intake from the analysis, the beta for each macronutrient represented the change in frailty index for a 5E% higher intake of that particular macronutrient and a concomitant lower intake of protein.
Based on previous literature [24, 25], three models were built: a basic model (model 1) adjusted for total energy intake (continuous), age (continuous), sex (categorical), length of follow-up (continuous), frailty index at baseline (continuous), and cohort (categorical). A confounder model (model 2) was additionally adjusted for education (categorical), smoking status (categorical), physical activity (continuous), income (categorical), living situation (categorical), occupational situation (categorical), and fiber and alcohol intake (in energy percentages, continuous) [37,38,39]. Finally, an intermediate model (model 3) was created which was additionally adjusted for BMI (continuous), because we hypothesized that BMI could be both a confounder and/or mediator in these associations. Analyses with specific subcategories of macronutrients were additionally adjusted for the other subcategories in energy percentages (e.g., animal protein was adjusted for vegetable protein intake and vice versa).
We performed several sensitivity analyses to test the robustness of the results. First, we applied the energy decomposition method to take total energy intake into account [40]. More details on this method are described elsewhere [36]. Second, effect modification was explored for age and sex [41,42,43], by adding interaction terms (macronutrient × effect modifier). Third, we excluded all deficits from the frailty index related to nutritional components (BMI, high-density lipoprotein, and hyperlipidemia) to evaluate if these deficits explained a possible association between macronutrients and frailty.
To impute missing values on the covariates, we constructed a multiple imputation procedure (n = 10 imputation sets). Results were presented by pooled analyses from multiple imputation data [44] and presented as betas (β) and 95% confidence intervals (95% CI). Statistical analyses were executed using IBM’s SPSS Statistics Version 24. Statistical tests were two-tailed.
Results
Characteristics of the study population
Of all 5205 participants, 59% were women and the median age of the population was 60 years (IQR 56; 63) (Table 1). The median energy intake was 2077 kcal (IQR 1727; 2511) of which, respectively, 44, 34, and 16 energy percentage of carbohydrate, fat, and protein. The median frailty index score at baseline was 13.8 points (IQR 9.6; 19.1), and on average, the frailty index increased by 2.3 points (IQR − 2.0; 7.6) after on average of 10.6 years of follow-up (range of 23 years). The frailty index at follow-up for the normal weight, overweight, and obese group was, respectively, 14.0 (8.8; 20.9), 15.6 (10.9; 22.3), and 19.7 (14.0; 27.5).
Macronutrients and frailty
By applying the nutrient residual method, after adjustment for confounders, total carbohydrate intake was not associated with frailty over time (Table 2) and also mono- and polysaccharides (Table 3) were not associated. Total fat, saturated, and poly-unsaturated fatty acids were not associated with frailty over time, but mono-unsaturated fatty acids was associated with more frailty over time in the total population [β 0.45 (95% CI 0.10; 0.81)]. Protein was associated, which was mainly by animal protein, with higher frailty levels over time, but only in the normal weight group [β 0.31 (95% CI 0.07; 0.56)] and not in the overweight or obese groups. The mediation model including BMI did not alter the results. By applying the nutrient density method, the direction of the associations remained mainly similar. A significant association between higher protein intake at the expense of carbohydrates and more frailty over time was observed [β 3.44 (95% CI 0.69; 6.19)], only in the normal weight group and not in the overweight or obese groups (Table 4). Also, a significant association was observed between higher protein intake at the expense of fat and more frailty over time [β 3.09 (95% CI 0.14; 6.04)] in the normal weight group, not in higher BMI groups.
Sensitivity analyses
First, we applied the energy decomposition method to take total energy intake into account (S1). In line with our main analyses, no associations were observed or carbohydrates or fat, and higher intake of protein was associated with more frailty over time in the normal weight group, but not in the overweight or obese groups [model 2: β 0.67 (95% CI 0.13; 1.21)]. Second, analyses were stratified based on significant interactions (p < 0.10). A significant interaction was observed between at least one of the macronutrients and sex (p value range 0.08–0.35), and no significant interaction was observed for age (p value range 0.12–0.37). Stratification by sex using the nutrient residual method did not alter the results (S2). Third, a sensitivity analysis excluding all nutritional components from the frailty index did not alter the direction or strength of the association of fat and protein with frailty (results not shown).
Discussion
This study did not observe an association between total carbohydrates and total fats with frailty over time. A positive association between mono-unsaturated fatty acids intake and frailty in the total population was observed. Furthermore, an association between protein intake and more frailty over time was seen, but only among those with normal weight. This association was mainly driven by animal protein which was associated with a higher frailty index score over time. Moreover, higher protein intake at the expense of a concomitant lower intake of carbohydrates or fat was associated with more frailty over time.
Comparison of our results with published data is challenging, because data on the association between nutrition and frailty are scarce. A recent review also emphasized that most studies focused on the association between protein intake and the physical domain of frailty [12]. Far less is known for other domains of frailty: cognition, mood, social health, and comorbidity. The frailty phenotype is physically orientated, and is distinct from disabilities, chronic diseases, cognition, and mental health, whereas the frailty index does include these health domains. Moreover, other studies used different definitions of frailty or overall health.
In our study, we did not find an association between carbohydrate intake and frailty after full adjustment. To our knowledge, no studies are known for assessing the association between carbohydrates and frailty. Furthermore, no association between overall total fat intake and frailty was seen in our study. Nevertheless, we did observe an association between mono-unsaturated fatty acids intake and more frailty over time in the total population. This result was unexpected as mono-unsaturated fatty acids are generally known to be beneficial for several components of frailty including cognition [45]. However, important contributors to total mono-unsaturated fatty acids intake are meat products, added fats, and dairy products [46]. In line with our results, Hodge et al. showed in a prospective cohort study that a dietary pattern, high meat, and fatty products were associated with worsening health [47].
We did not observe an association between total protein intake and frailty in the full population. The possible beneficial effect of high protein intake on muscle function may be omitted by a possible negative association between protein and other health domains including digestive, renal, and vascular domains [48]. Also, high dietary protein intake is often associated with a low diet quality, which might have a negative effect on the frailty status [23,24,25, 49]. In our study, we did observe an association between high intake of protein at the expense of carbohydrates and more frailty over time. This is in line with two systematic reviews which concluded that high protein intake but a low carbohydrate intake was associated with higher all-cause mortality risk [19, 20]. Also, we did observe an association between higher protein intake and increased frailty scores among participants with a normal weight, but not in participants who were overweighed or obese. High protein diet is associated with lower food intake, lower body weight, and body adiposity [14,15,16,17]; this might explain that we did observe an association in normal weight participants, but not in overweight or obese. Persons with overweight or obesity have in general a high nutritional intake and, therefore, comply with dietary guidelines; however, the macronutrient composition might be less important for older adults suffering from overweight or obesity as an overall unhealthy diet mediates the association between the macronutrient composition and frailty. In our study, the association between protein and higher frailty status over time is mainly driven by higher intake of animal protein. A diet high in animal protein intake (such as meat) contributes to a higher dietary acid load. Because a high dietary acid load is associated with different chronic diseases, this might contribute to a higher frailty index score [50, 51]. Whereas high intake of plant protein is associated with a healthy dietary pattern which is, in turn, associated with a lower frailty status [24, 25, 27, 52].
This study has numerous strengths. To the best of our knowledge, this is the first study investigating the longitudinal association between macronutrient intake and the frailty index. Additionally, the comprehensive data collection allowed us to control for many confounders. Furthermore, the large sample size and multiple imputation procedure contributed to a more precise estimate of the association. Most studies on protein did not take into account the role of energy intake and other macronutrients in the diet and it is, therefore, unclear whether the onset and progression of frailty is affected by higher absolute or relative intake of protein, and for relative measure, if this is explained by lower intake of carbohydrates or fat. By taking total energy intake into account, the interpretation of the role of specific macronutrients will improve [40]. The present study used different statistical methods to take the possible modifying and confounding effect of total energy into account, giving us more insight into the association between macronutrient intakes and frailty.
Despite these strengths, there are several limitations to consider. First, since there is no consensus on the definition of frailty, there are a variety of instruments to assess frailty and overall health which limits the comparability of our results. Measures of frailty show important differences with the frailty index, making a direct comparison with previous literature complex. Second, participants had relatively low frailty indices, and in many participants (37%), the frailty index became lower over time, whereas it was expected to increase. This might be explained, because a relatively healthy population participated in this study, which might have been expected as older adults who are frail or more vulnerable are less likely to participate in the study [53,54,55]. This may have led to less strong associations. This limits the generalizability of our study results in more vulnerable populations. Third, because this study included multiple waves of the Rotterdam Study, different FFQs were used to measure dietary intake. Nevertheless, the use of an up-to-date FFQ to assess dietary intake has been advised to take into account the availability of new foods and new food composition [56]. Finally, results may have been influenced by report bias as persons may give more socially desirable answers and exaggerate the consumption of healthy foods which might increase our estimate of the effect [57].
In conclusion, our study contributed to the knowledge on the association between macronutrients and frailty over time. The intake of fat and carbohydrates did not contribute to the association between the macronutrient composition of the diet and overall health, measured by the frailty index. High protein intake, specifically animal protein intake, is associated with more frailty in a relatively healthy older adult population. Further research is needed on the association between protein intake and multidimensional frailty, focused on the source of protein, and on more vulnerable populations.
References
Beard JR, Bloom DE (2015) Towards a comprehensive public health response to population ageing. Lancet 385:658–661
DuGoff EH, Canudas-Romo V, Buttorff C, Leff B, Anderson GF (2014) Multiple chronic conditions and life expectancy: a life table analysis. Med Care 52:688–694
Mitnitski AB, Mogilner AJ, Rockwood K (2001) Accumulation of deficits as a proxy measure of aging. Sci World J 1:323–336
Gobbens RJ, van Assen MA, Luijkx KG, Wijnen-Sponselee MT, Schols JM (2010) Determinants of frailty. J Am Med Dir Assoc 11:356–364
Fried LP, Tangen CM, Walston J et al (2001) Frailty in older adults evidence for a phenotype. J Gerontol Ser A Biol Sci Med Sci 56:M146–M157
Walston J, Hadley EC, Ferrucci L, Guralnik JM, Newman AB et al (2006) Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc 54:991–1001
Gobbens R, Luijkx KG, Wijnen-Sponselee MT, van Assen MALM, Schols JMGA (2011) Wetenschappelijke definities en metingen van kwetsbaarheid. In Kwetsbare ouderen. Sociaal en Cultureel Planbureau, Den Haag, pp 39–48
Lahousse L, Maes B, Ziere G et al (2014) Adverse outcomes of frailty in the elderly: the Rotterdam Study. Eur J Epidemiol 29:419–427
Searle SD, Minitski A, Gahbauer EA et al (2008) A standard procedure for creating a frailty index. BMC Geriatr 8:24
Kulminski A, Ukraintseva SV, Akushevich I, Arbeev KG, Land K et al (2007) Accelerated accumulation of health deficits as a characteristic of aging. Exp Gerontol 42:963–970
Goisser S, Guyonnet S, Volkert D (2016) The Role of nutrition in frailty: an overview. J Frailty Aging 5:74–77
Schoufour JD, Overdevest E, Weijs PJM, Tieland M (2019) Dietary protein, exercise, and frailty domains. Nutrients 11(10):E2399
Wolfe RR (2012) The role of dietary protein in optimizing muscle mass, function and health outcomes in older individuals. Br J Nutr 108(Suppl 2):S88–S93
Le Couteur DG, Solon-Biet S, Cogger VC, Mitchell SJ, Senior A et al (2016) The impact of low-protein high-carbohydrate diets on aging and lifespan. Cell Mol Life Sci 73:1237–1252
Westerterp-Plantenga MS, Lemmens SG, Westerterp KR (2012) Dietary protein - its role in satiety, energetics, weight loss and health. Br J Nutr 108(Suppl 2):S105–S112
Ajala O, English P, Pinkney J (2013) Systematic review and meta-analysis of different dietary approaches to the management of type 2 diabetes. Am J Clin Nutr 97:505–516
Gardner CD, Kiazand A, Alhassan S, Kim S, Stafford RS et al (2007) Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: the A TO Z Weight Loss Study: a randomized trial. J Am Med Assoc 297:969–977
Juraschek SP, Appel LJ, Anderson CA, Miller ER (2013) Effect of a high-protein diet on kidney function in healthy adults: results from the OmniHeart trial. Am J Kidney Dis 61:547–554
Noto H, Goto A, Tsujimoto T, Noda M (2013) Low-carbohydrate diets and all-cause mortality: a systematic review and meta-analysis of observational studies. PLoS One 8:e55030
Pedersen AN, Kondrup J, Borsheim E (2013) Health effects of protein intake in healthy adults: a systematic literature review. Food Nutr Res. https://doi.org/10.3402/fnr.v57i0.21245
Hou L, Li F, Wang Y, Ou Z, Xu D et al (2015) Association between dietary patterns and coronary heart disease: a meta-analysis of prospective cohort studies. Int J Clin Exp Med 8:781–790
Flegal KM, Kit BK, Orpana H, Graubard BI (2013) Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. J Am Med Assoc 309:71–82
Woo J, Chan R, Leung J, Wong M (2010) Relative contributions of geographic, socioeconomic, and lifestyle factors to quality of life, frailty, and mortality in elderly. PLoS One 5:e8775
Brinkman S, Voortman T, Kiefte-de Jong JC, van Rooij FJA, Ikram MA et al (2017) The association between lifestyle and overall health, using the frailty index: the Rotterdam Study. Arch Gerontol Geriatr 76:85–91
De Haas SCM, De Jonge EAL, Voortman T, Graaff JS, Franco OH et al (2018) Dietary patterns and changes in Frailty status—the Rotterdam Study. Eur J Nutr 57(7):2365–2375
Ikram MA, Brusselle GGO, Murad SD, van Duijn CM, Franco OH et al (2017) The Rotterdam Study: 2018 update on objectives, design and main results. Eur J Epidemiol 32:807–850
Voortman T, Kiefte-de Jong JC, Ikram MA, Stricker BH, van Rooij FJA et al (2017) Adherence to the 2015 Dutch dietary guidelines and risk of non-communicable diseases and mortality in the Rotterdam Study. Eur J Epidemiol 32:993–1005
Klipstein-Grobusch K, den Breeijen JH, Goldbohm RA, Geleijnse JM, Hofman A et al (1998) Dietary assessment in the elderly: validation of a semiquantitative food frequency questionnaire. Eur J Clin Nutr 52:588–596
Goldbohm RA, van den Brandt PA, Brants HA, van’t Veer P, Al M et al (1994) Validation of a dietary questionnaire used in a large-scale prospective cohort study on diet and cancer. Eur J Clin Nutr 48:253–265
Stichting Nederlands Voedingsstoffenbestand (2006) NEVO-tabel: Nederlands Voedingsstoffenbestand 2006. Nederlands voedingsstoffenbestand, NEVO tabel
Schoufour JD, Erler NS, Jaspers L, Kiefte-deJong JC, Voortman T et al (2017) Design of a frailty index among community living middle-aged and older people: the Rotterdam study. Maturitas 97:14–20
Caspersen CJ, Bloemberg BP, Saris WH, Merritt RK, Kromhout D (1991) The prevalence of selected physical activities and their relation with coronary heart disease risk factors in elderly men: the Zutphen Study, 1985. Am J Epidemiol 133:1078–1092
Stel VS, Smit JH, Pluijm SM, Visser M, Deeg DJ et al (2004) Comparison of the LASA Physical Activity Questionnaire with a 7-day diary and pedometer. J Clin Epidemiol 57:252–258
Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR et al (2011) 2011 Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc 43:1575–1581
Ruhunuhewa I, Adjibade M, Andreeva VA, Galan P, Hercberg S et al (2017) Prospective association between body mass index at midlife and healthy aging among French adults. Obesity (Silver Spring) 25(7):1254–1262
Willett WC, Howe GR, Kushi LH (1997) Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr 65:1220S–1228S (discussion 1229S–1231S)
Srikanthan P, Seeman TE, Karlamangla AS (2009) Waist-hip-ratio as a predictor of all-cause mortality in high-functioning older adults. Ann Epidemiol 19:724–731
Sandoval-Insausti H, Perez-Tasigchana RF, Lopez-Garcia E, Garcia-Esquinas E, Rodriguez-Artalejo F et al (2016) Macronutrients intake and incident frailty in older adults: a prospective cohort study. J Gerontol Ser A Biol Sci Med Sci 71:1329–1334
Shikany JM, Barrett-Connor E, Ensrud KE, Cawthon PM, Lewis CE et al (2014) Macronutrients, diet quality, and frailty in older men. J Gerontol Ser A Biol Sci Med Sci 69:695–701
Willett W, Stampfer MJ (1986) Total energy intake: implications for epidemiologic analyses. Am J Epidemiol 124(1):17–27
Lindahl-Jacobsen R, Hanson HA, Oksuzyan A, Mineau GP, Christensen K et al (2013) The male–female health-survival paradox and sex differences in cohort life expectancy in Utah, Denmark, and Sweden 1850–1910. Ann Epidemiol 23:161–166
Romero-Ortuno R, Fouweather T, Jagger C (2013) Cross-national disparities in sex differences in life expectancy with and without frailty. Age Ageing 43:222–228
El Khoudary SR, McClure CK, VoPham T, Karvonen-Gutierrez CA, Sternfeld B et al (2014) Longitudinal assessment of the menopausal transition, endogenous sex hormones, and perception of physical functioning: the study of women’s health across the nation. J Gerontol Ser A 69:1011–1017
Donders AR, van der Heijden GJ, Stijnen T, Moons KG (2006) Review: a gentle introduction to imputation of missing values. J Clin Epidemiol 59:1087–1091
Solfrizzi V, Colacicco AM, D’Introno A, Capurso C, Torres F et al (2006) Dietary intake of unsaturated fatty acids and age-related cognitive decline: a 8.5-year follow-up of the Italian Longitudinal Study on Aging. Neurobiol Aging 27:1694–1704
Linseisen J, Welch AA, Ocke M, Amiano P, Agnoli C et al (2009) Dietary fat intake in the European Prospective Investigation into Cancer and Nutrition: results from the 24-h dietary recalls. Eur J Clin Nutr 63(Suppl 4):S61–S80
Hodge AM, O’Dea K, English DR, Flicker L (2014) Dietary patterns as predictors of successful ageing. J Nutr Health Aging 18:221–227
Wu G (2016) Dietary protein intake and human health. Food Funct 7:1251–1265
de Vries NM, Staal JB, van Ravensberg CD, Hobbelen JS, Olde Rikkert MG et al (2011) Outcome instruments to measure frailty: a systematic review. Ageing Res Rev 10:104–114
Xu H, Akesson A, Orsini N, Hakansson N, Wolk A et al (2016) Modest U-shaped association between dietary acid load and risk of all-cause and cardiovascular mortality in adults. J Nutr 146:1580–1585
Kiefte-de Jong JC, Li Y, Chen M, Curhan GC, Mattei J et al (2017) Diet-dependent acid load and type 2 diabetes: pooled results from three prospective cohort studies. Diabetologia 60:270–279
Schoufour JD, Franco OH, Kiefte-de Jong JC, Trajanoska K, Stricker B et al (2018) The association between dietary protein intake, energy intake and physical frailty: results from the Rotterdam Study. Br J Nutr 121:393–401
Hoffmann K, Schulze MB, Schienkiewitz A, Nöthlings U, Boeing H (2004) Application of a new statistical method to derive dietary patterns in nutritional epidemiology. Am J Epidemiol 159:935–944
Delgado-Rodriguez M, Llorca J (2004) Bias. J Epidemiol Community Health 58:635–641
Leening MJG, Heeringa J, Deckers JW, Franco OH, Hofman A et al (2014) Healthy volunteer effect and cardiovascular risk. Epidemiology 25:470–471
Cade J, Thompson R, Burley V, Warm D (2002) Development, validation and utilisation of food-frequency questionnaires—a review. Public Health Nutr 5:567–587
Schoufour JD, de Jonge EAL, Kiefte-de Jong JC, van Lenthe FJ, Hofman A et al (2018) Socio-economic indicators and diet quality in an older population. Maturitas 107:71–77
Acknowledgements
The authors are grateful to the study participants, the staff from the Rotterdam Study, and the participating general practitioners and pharmacists. The Rotterdam Study is funded by Erasmus Medical Center and Erasmus University, Rotterdam, Netherlands Organization for the Health Research and Development (ZonMw), the Research Institute for Diseases in the Elderly (RIDE), the Ministry of Education, Culture and Science, the Ministry for Health, Welfare and Sports, the European Commission (DG XII), and the Municipality of Rotterdam. Fernando Rivadeneira received a grant from the Netherlands Organization for Health Research and Development ZonMw VIDI 016.136.367. The funders played no role in the study design or in data collection and analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
The RS was conducted according to the Declaration of Helsinki and all procedures involving human subjects were approved by the Medical Ethics Committee of the Erasmus MC and by the Ministry of Health, Welfare and Sport of the Netherlands, implementing the Wet Bevolkingsonderzoek: ERGO (Population Studies Act: Rotterdam Study). All persons gave their informed consent prior to their inclusion in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Verspoor, E., Voortman, T., van Rooij, F.J.A. et al. Macronutrient intake and frailty: the Rotterdam Study. Eur J Nutr 59, 2919–2928 (2020). https://doi.org/10.1007/s00394-019-02131-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-019-02131-0