Abstract
Background
Numerous epidemiological and animal studies have shown that consumption of red wine is related to reduced incidence of cardiovascular diseases and cancer. Trans-resveratrol (3, 5, 4′-trihydroxystilbene), a phenolic compound present in wine, has been reported to have a potential cancer chemopreventive activity. Moreover, it may exert a protective effect against atherogenesis through its antioxidant properties. Trans-piceid (3-ß glucoside of trans-resveratrol) is present to a greater extent than its aglycone in red wine, but hydrolysis of this glycosylated derivative can occur in small intestine and liver, which would enhance the amount of the biological active trans-resveratrol.
Aims
The present study aimed to investigate the rate of transepithelial transport of trans-piceid using human intestinal Caco-2 cell monolayers and metabolism of this compound during its absorption across the small intestine.
Methods
The transport of trans-piceid was evaluated in the human epithelial cell line Caco-2, which possesses enterocyte-like properties in vitro. For transepithelial experiments, confluent monolayers of Caco-2 cells were grown on Transwell® inserts. For metabolic studies, we used both Caco-2 cells seeded on 6-well plates and rat small intestine cell-free extracts.
Results
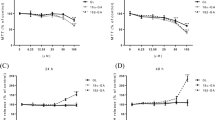
The time course of apical (AP) to basolateral (BL) transport of trans-piceid showed that the favorable apparent permeability coefficient (Papp) declined rapidly during the 6 h of the experiment. This observation could be correlated with the appearance of metabolites. After incubation of Caco-2 cells with trans-piceid, trans-resveratrol was detected on both AP and BL sides. By using protein extracts obtained from rat, we conclude that the Lactase Phlorizin Hydrolase (LPH) and Cytosolic-ß-Glucosidase (CBG) are involved in the hydrolysis of trans-piceid. Furthermore, we show that after deglycosylation, the resulting aglycone is metabolized in trans-resveratrol-3-O-ß-glucuronide and to a lesser extent in trans-resveratrol-4′-O-ß-glucuronide, and that UGT1A1 is mainly involved in this metabolism.
Conclusions
This study demonstrates that the transepithelial transport of trans-piceid occurs at a high rate and that the compound is deglycosylated in trans-resveratrol. There are two possible pathways by which trans-piceid is hydrolyzed in the intestine. The first is a cleavage by the CBG, after passing the brush-border membrane by SGLT1. The second is deglycosylation on the luminal side of the epithelium by the membrane-bound enzyme LPH, followed by passive diffusion of the released aglycone, which is further metabolized inside the cells into two glucuronoconjugates.



Similar content being viewed by others
References
Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC, Pezzuto JM (1997) Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 275:218–220
Vitrac X, Monti JP, Vercauteren J, Deffieux G, Merillon JM (2002) Direct liquid chromatographic analysis of resveratrol derivatives and flavanonols in wines with absorbance and fluorescence detection. Anal Chim Acta 458:103–110
Silberberg M, Morand C, Mathevon T, Besson C, Manach C, Scalbert A, Remesy C (2006) The bioavailability of polyphenols is highly governed by the capacity of the intestine and of the liver to secrete conjugated metabolites. Eur J Nutr 45:88–96
Vitrac X, Desmoulière A, Brouillaud B, Krisa S, Deffieux G, Barthe N, Rosenbaum J, Merillon JM (2003) Distribution of [14C]-trans-resveratrol, a cancer chemopreventive polyphenol, in mouse tissues after oral administration. Life Sci 72:2219–2233
Aumont V, Krisa S, Battaglia E, Netter P, Richard T, Merillon JM, Magdalou J, Sabolovic N (2001) Regioselective and stereospecific glucuronidation of trans- and cis-resveratrol in human. Arch Biochem Biophys 393:281–289
Kuhnle G, Spencer J, Chowrimootoo G, Schroeter H, Debnam E, Srai KS, Rice-Evans C, Hahn U (2000) Resveratrol is absorbed in the small intestine as resveratrol glucuronide. Biochem Biophys Res Commun 272:212–217
Artursson P, Karlsson J (1991) Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem Biophys Res Commun 175:880–885
Walgren RA, Karnaky KJ, Lindenmayer GE, Walle T (2000) Efflux of dietary flavonoid quercetin 4′-beta-glucoside across human intestinal Caco-2 cell monolayers by apical multidrug resistance-associated protein-2. J Pharmacol Exp Ther 294:830–836
Henry C, Vitrac X, Decendit A, Ennamany R, Krisa S, Merillon JM (2005) Cellular uptake and efflux of trans-piceid and its aglycone trans-resveratrol on the apical membrane of human intestinal Caco-2 cells. J Agric Food Chem 53:798–803
Waffo Téguo P, Decendit A, Vercauteren J, Deffieux G, Merillon JM (1996) Trans-Resveratrol-3-O-beta-Glucoside (Piceid) in cell suspension cultures of Vitis vinifera. Psychochemistry 42: 1591–1593
Nemeth K, Plumb GW, Berrin JG, Juge N, Jacob R, Naim HY, Williamson G, Swallow DM, Kroon PA (2003) Deglycosylation by small intestinal epithelial cell beta-glucosidases is a critical step in the absorption and metabolism of dietary flavonoid glycosides in humans. Eur J Nutr 42:29–42
Yee S (1997) In vitro permeability across Caco-2 cells (colonic) can predict in vivo (small intestinal) absorption in man – fact or myth. Pharm Res 14:763–766
Walle UK, Galijatovic A, Walle T (1999) Transport of the flavonoid chrysin and its conjugated metabolites by the human intestinal cell line Caco-2. Biochem Pharmacol 58:431–438
Day AJ, Gee JM, DuPont MS, Johnson IT, Williamson G (2003) Absorption of quercetin-3-glucoside and quercetin-4′-glucoside in the rat small intestine: the role of lactase phlorizin hydrolase and the sodium-dependent glucose transporter. Biochem Pharmacol 65:1199–1206
Galijatovic A, Otake Y, Walle UK, Walle T (2001) Induction of UDP-glucuronosyltransferase UGT1A1 by the flavonoid chrysin in Caco-2 cells – potential role in carcinogen bioinactivation. Pharm Res 18:374–379
Wilkinson AP, Gee JM, Dupont MS, Needs PW, Mellon FA, Williamson G, Johnson IT (2003) Hydrolysis by lactase phlorizin hydrolase is the first step in the uptake of daidzein glucosides by rat small intestine in vitro. Xenobiotica 33:255–264
Walgren RA, Lin JT, Kinne RK, Walle T (2000) Cellular uptake of dietary flavonoid quercetin 4′-beta-glucoside by sodium-dependent glucose transporter SGLT1. J Pharmacol Exp Ther 294:837–843
Chen J, Lin H, Hu M (2005) Absorption and metabolism of genistein and its five isoflavone analogs in the human intestinal Caco-2 model. Cancer Chemother Pharmacol. 55:159–169
Ng SP, Wong KY, Zhang L, Zuo Z, Lin G (2004) Evaluation of the first-pass glucuronidation of selected flavones in gut by Caco-2 monolayer model. J Pharm Pharm Sci 8:1–9
Walle UK, Walle T (2002) Induction of human UDP-glucuronosyltransferase UGT1A1 by flavonoids-structural requirements. Drug Metab Disp 30:564–569
Kaldas MI, Walle UK, Walle T (2003) Resveratrol transport and metabolism by human intestinal Caco-2 cells. J Pharm Pharmacol 55:307–312
Li Y, Shin YG, Yu C, Kosmeder JW, Hirschelman WH, Pezzuto JM, van Breemen RB (2003) Increasing the throughput and productivity of Caco-2 cell permeability assays using liquid chromatography-mass spectrometry: application to resveratrol absorption and metabolism. Comb Chem High Throughput Screen 6:757–767
Williamson G, Day AJ, Plumb GW, Couteau D (2000) Human metabolic pathways of dietary flavonoids and cinnamates. Biochem Soc Trans 28:16–22
Walle UK, French KL, Walgren RA, Walle T (1999) Transport of genistein-7-glucoside by human intestinal Caco-2 cells: potential role for MRP2. Res Commun Mol Pathol Pharmacol 103:45–46
Sergent T, Garsou S, Schaut A, Saeger SD, Pussemier L, Peteghem CV, Larondelle Y, Schneider YJ (2005) Differential modulation of ochratoxin A absorption across Caco-2 cells by dietary polyphenols, used at realistic intestinal concentrations. Toxicol Lett 159:60–70
Sperker B, Backman JT, Kroemer HK (1997) The role of beta-glucuronidase in drug disposition and drug targeting in humans. Clin Pharmacokinet 33:18–31
Acknowledgments
This study was supported by a grant from the Région Aquitaine, France. We are grateful to Dr. Ray Cooke for reading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Henry-Vitrac, C., Desmoulière, A., Girard, D. et al. Transport, deglycosylation, and metabolism of trans-piceid by small intestinal epithelial cells. Eur J Nutr 45, 376–382 (2006). https://doi.org/10.1007/s00394-006-0609-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-006-0609-8