Abstract
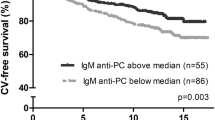
Low anti-phosphorylcholine (PC) IgM plasma levels have been associated with increased incidence of adverse events in coronary artery disease (CAD). The underlying mechanisms are unclear. We hypothesized that atheroprotection mediated by anti-PC IgM antibodies is associated with reduced vascular remodeling and therefore tested whether anti-PC IgM plasma levels independently predict vascular remodeling. In a prospective cross-sectional study, anti-PC IgM plasma levels were measured in 175 consecutive patients with suspected CAD undergoing cardiac computed tomography angiography. Plaque morphology was thoroughly analyzed. Vascular remodeling was defined by a change in the vessel diameter at the plaque site in comparison to the reference segment proximal to the lesion (reference diameter) of ≥10 %. Mean age of the patients was 64.8 ± 10.7 years, 48.6 % were female. In 98 patients CAD was diagnosed, 57 (58.2 %) of which displayed non-obstructive CAD (stenosis <50 %), whereas 41 (41.8 %) exhibited obstructive CAD (stenosis ≥50 %). In 34 of 98 (34.7 %) CAD patients vascular remodeling was present. Mean anti-PC IgM levels did not differ between patients with and without CAD (70.8 ± 52.7 vs. 69.1 ± 55.1 U/mL). However, anti-PC IgM levels were significantly lower in CAD patients compared to those without vascular remodeling (46.6 ± 31.6 vs. 73.3 ± 58.5 U/mL, P = 0.024). Using multivariate logistic regression, anti-PC IgM plasma levels independently predicted coronary vascular remodeling (HR 0.322, 95 % confidence interval 0.121–0.856, P = 0.023). In conclusion, low anti-PC IgM levels are independently associated with coronary vascular remodeling. These findings may represent the link between in vitro studies demonstrating atheroprotective effects of anti-PC IgM and clinical data demonstrating that low anti-PC IgM levels are associated with adverse outcome in CAD patients.

Similar content being viewed by others
References
Ajeganova S, Ehrnfelt C, Alizadeh R, Rohani M, Jogestrand T, Hafström I, Frostegård J (2011) Longitudinal levels of apolipoproteins and antibodies against phosphorylcholine are independently associated with carotid artery atherosclerosis 5 years after rheumatoid arthritis onset—a prospective cohort study. Rheumatology 50:1785–1793. doi:10.1093/rheumatology/ker204
Andrassy M, Volz HC, Schuessler A, Gitsioudis G, Hofmann N, Laohachewin D, Wienbrandt AR, Kaya Z, Bierhaus A, Giannitsis E, Katus HA, Korosoglou G (2012) HMGB1 is associated with atherosclerotic plaque composition and burden in patients with stable coronary artery disease. PLoS One 7:e52081. doi:10.1371/journal.pone.0052081
Binder C (2010) Natural IgM antibodies against oxidation-specific epitopes. J Clin Immunol 30:56–60. doi:10.1007/s10875-010-9396-3
Bischoff B, Hein F, Meyer T, Hadamitzky M, Martinoff S, Schomig A, Hausleiter J (2009) Impact of a reduced tube voltage on CT angiography and radiation dose: results of the PROTECTION I study. JACC Cardiovasc Imaging 2:940–946. doi:10.1016/j.jcmg.2009.02.015
Caidahl K, Hartford M, Karlsson T, Herlitz J, Pettersson K, de Faire U, Frostegård J (2013) IgM-phosphorylcholine autoantibodies and outcome in acute coronary syndromes. Int J Cardiol 167:464–469. doi:10.1016/j.ijcard.2012.01.018
Caligiuri G, Khallou-Laschet J, Vandaele M, Gaston A-T, Delignat S, Mandet C, Kohler HV, Kaveri SV, Nicoletti A (2007) Phosphorylcholine-targeting immunization reduces atherosclerosis. J Am Coll Cardiol 50:540–546
Cho HJ, Shashkin P, Gleissner CA, Dunson D, Jain N, Lee JK, Miller Y, Ley K (2007) Induction of dendritic cell-like phenotype in macrophages during foam cell formation. Physiol Genomics 29:149–160
de Faire U, Frostegård J (2009) Natural antibodies against phosphorylcholine in cardiovascular disease. Ann N Y Acad Sci 1173:292–300. doi:10.1111/j.1749-6632.2009.04748.x
de Faire U, Su J, Hua X, Frostegård A, Halldin M, Hellenius M-L, Wikström M, Dahlbom I, Grönlund H, Frostegård J (2010) Low levels of IgM antibodies to phosphorylcholine predict cardiovascular disease in 60-year old men: Effects on uptake of oxidized LDL in macrophages as a potential mechanism. J Autoimmun 34:73–79. doi:10.1016/j.jaut.2009.05.003
De Metrio M, Milazzo V, Marenzi G (2012) Serum vitamin D concentration status and its correlation with early biomarkers of remodeling following acute myocardial infarction. Clin Res Cardiol Off J Ger Card Soc 101:771–772. doi:10.1007/s00392-012-0457-x
Faria-Neto JR, Chyu K-Y, Li X, Dimayuga PC, Ferreira C, Yano J, Cercek B, Shah PK (2006) Passive immunization with monoclonal IgM antibodies against phosphorylcholine reduces accelerated vein graft atherosclerosis in apolipoprotein E-null mice. Atherosclerosis 189:83–90
Ferencik M, Schlett CL, Ghoshhajra BB, Kriegel MF, Joshi SB, Maurovich-Horvat P, Rogers IS, Banerji D, Bamberg F, Truong QA, Brady TJ, Nagurney JT, Hoffmann U (2012) A computed tomography-based coronary lesion score to predict acute coronary syndrome among patients with acute chest pain and significant coronary stenosis on coronary computed tomographic angiogram. Am J Cardiol 110:183–189. doi:10.1016/j.amjcard.2012.02.066
Fischer-Rasokat U, Honold J, Seeger FH, Fichtlscherer S, Schachinger V, Dimmeler S, Zeiher AM, Assmus B (2012) Early remodeling processes as predictors of diastolic function 5 years after reperfused acute myocardial infarction and intracoronary progenitor cell application. Clin Res Cardiol Off J Ger Card Soc 101:209–216. doi:10.1007/s00392-011-0382-4
Fiskesund R, Stegmayr B, Hallmans G, Vikström M, Weinehall L, de Faire U, Frostegård J (2010) Low levels of antibodies against phosphorylcholine predict development of stroke in a population-based study from Northern Sweden. Stroke 41:607–612. doi:10.1161/strokeaha.109.558742
Galkina EV, Ley K (2009) Immune and inflammatory mechanisms of atherosclerosis. Annu Rev Immunol 27:165–197. doi:10.1146/annurev.immunol.021908
Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ (1987) Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med 316:1371–1375
Glass CK, Witztum JL (2001) Atherosclerosis. The road ahead. Cell 104:503–516
Gleissner CA, Leitinger N, Ley K (2007) Effects of native and modified low-density lipoproteins on monocyte recruitment in atherosclerosis. Hypertension 50:276–283. doi:10.1161/HYPERTENSIONAHA.107.089854
Gleissner CA, Sanders JM, Nadler J, Ley K (2008) Upregulation of aldose reductase during foam cell formation as possible link among diabetes, hyperlipidemia, and atherosclerosis. Arterioscler Thromb Vasc Biol 28:1137–1143. doi:10.1161/ATVBAHA.107.158295
Grönlund H, Hallmans G, Jansson JH, Boman K, Wikström M, de Faire U, Frostegård J (2009) Low levels of IgM antibodies against phosphorylcholine predict development of acute myocardial infarction in a population-based cohort from northern Sweden. Eur J Cardiovasc Prev Rehabil 16:382–386. doi:10.1097/HJR.0b013e32832a05df
Grundy SM, Cleeman JI, Merz CN, Brewer HB Jr, Clark LT, Hunninghake DB, Pasternak RC, Smith SC Jr, Stone NJ (2004) Implications of recent clinical trials for the National cholesterol education program adult treatment panel III guidelines. Circulation 110:227–239
Hadamitzky M, Distler R, Meyer T, Hein F, Kastrati A, Martinoff S, Schömig A, Hausleiter J (2011) Prognostic Value of Coronary Computed Tomographic Angiography in Comparison With Calcium Scoring and Clinical Risk Scores. Circ Cardiovasc Imaging 4:16–23. doi:10.1161/circimaging.110.955351
Hernando L, Corros C, Gonzalo N, Hernandez-Antolin R, Banuelos C, Jimenez-Quevedo P, Bernardo E, Fernandez-Ortiz A, Escaned J, Macaya C, Alfonso F (2013) Morphological characteristics of culprit coronary lesions according to clinical presentation: insights from a multimodality imaging approach. Int J Cardiovasc Imaging 29:13–21. doi:10.1007/s10554-012-0043-3
Hoefer IE, Sels JW, Jukema JW, Bergheanu S, Biessen E, McClellan E, Daemen M, Doevendans P, de Groot P, Hillaert M, Horsman S, Ilhan M, Kuiper J, Pijls N, Redekop K, van der Spek P, Stubbs A, van de Veer E, Waltenberger J, van Zonneveld AJ, Pasterkamp G (2013) Circulating cells as predictors of secondary manifestations of cardiovascular disease: design of the CIRCULATING CELLS study. Clin Res Cardiol Off J Ger Card Soc 102:847–856. doi:10.1007/s00392-013-0607-9
Hoffmann U, Moselewski F, Nieman K, Jang I-K, Ferencik M, Rahman AM, Cury RC, Abbara S, Joneidi-Jafari H, Achenbach S, Brady TJ (2006) Noninvasive assessment of plaque morphology and composition in culprit and stable lesions in acute coronary syndrome and stable lesions in stable angina by multidetector computed tomography. J Am Coll Cardiol 47:1655–1662
Kearney JF (2000) Immune recognition of OxLDL in atherosclerosis. J Clin Investig 105:1683–1685. doi:10.1172/JCI10426
Khalili H, Talasaz AH, Salarifar M (2012) Serum vitamin D concentration status and its correlation with early biomarkers of remodeling following acute myocardial infarction. Clin Res Cardiol Off J Ger Card Soc 101:321–327. doi:10.1007/s00392-011-0394-0
Korosoglou G, Lehrke S, Mueller D, Hosch W, Kauczor HU, Humpert PM, Giannitsis E, Katus HA (2011) Determinants of troponin release in patients with stable coronary artery disease: insights from CT angiography characteristics of atherosclerotic plaque. Heart 97:823–831. doi:10.1136/hrt.2010.193201
Korosoglou G, Mueller D, Lehrke S, Steen H, Hosch W, Heye T, Kauczor H-U, Giannitsis E, Katus H (2010) Quantitative assessment of stenosis severity and atherosclerotic plaque composition using 256-slice computed tomography. Eur Radiol 20:1841–1850. doi:10.1007/s00330-010-1753-3
Kroner ES, van Velzen JE, Boogers MJ, Siebelink HM, Schalij MJ, Kroft LJ, de Roos A, van der Wall EE, Jukema JW, Reiber JH, Schuijf JD, Bax JJ (2011) Positive remodeling on coronary computed tomography as a marker for plaque vulnerability on virtual histology intravascular ultrasound. Am J Cardiol 107:1725–1729. doi:10.1016/j.amjcard.2011.02.337
Lewis MJ, Malik TH, Ehrenstein MR, Boyle JJ, Botto M, Haskard DO (2009) Immunoglobulin M is required for protection against atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation 120:417–426. doi:10.1161/circulationaha.109.868158
Lok DJ, Lok SI, Bruggink-Andre de la Porte PW, Badings E, Lipsic E, van Wijngaarden J, de Boer RA, van Veldhuisen DJ, van der Meer P (2013) Galectin-3 is an independent marker for ventricular remodeling and mortality in patients with chronic heart failure. Clin Res Cardiol Off J Ger Card Soc 102:103–110. doi:10.1007/s00392-012-0500-y
Motoyama S, Kondo T, Sarai M, Sugiura A, Harigaya H, Sato T, Inoue K, Okumura M, Ishii J, Anno H, Virmani R, Ozaki Y, Hishida H, Narula J (2007) Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J Am Coll Cardiol 50:319–326
Motoyama S, Sarai M, Harigaya H, Anno H, Inoue K, Hara T, Naruse H, Ishii J, Hishida H, Wong ND, Virmani R, Kondo T, Ozaki Y, Narula J (2009) Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol 54:49–57. doi:10.1016/j.jacc.2009.02.068
Palinski W, Hörkkö S, Miller E, Steinbrecher UP, Powell HC, Curtiss LK, Witztum JL (1996) Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasma. J Clin Investig 98:800–814. doi:10.1172/JCI118853
Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH (1997) Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med 336:973–979. doi:10.1056/NEJM199704033361401
Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, Carnethon MR, Dai S, de Simone G, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Greenlund KJ, Hailpern SM, Heit JA, Ho PM, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, McDermott MM, Meigs JB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Rosamond WD, Sorlie PD, Stafford RS, Turan TN, Turner MB, Wong ND, Wylie-Rosett J, Committee obotAHAS, Stroke Statistics Subcommittee, Disease ObotAHAH, Stroke Statistics Writing Group (2011) Heart disease and stroke statistics—2011 Update: a report from the American Heart Association. Circulation 123:e18–209. doi:10.1161/CIR.0b013e3182009701
Shashkin P, Dragulev B, Ley K (2005) Macrophage differentiation to foam cells. Curr Pharm Des 11:3061–3072
Shmilovich H, Cheng VY, Tamarappoo BK, Dey D, Nakazato R, Gransar H, Thomson LE, Hayes SW, Friedman JD, Germano G, Slomka PJ, Berman DS (2011) Vulnerable plaque features on coronary CT angiography as markers of inducible regional myocardial hypoperfusion from severe coronary artery stenoses. Atherosclerosis 219:588–595. doi:10.1016/j.atherosclerosis.2011.07.128
Sinning C, Kieback A, Wild PS, Schnabel RB, Ojeda F, Appelbaum S, Zeller T, Lubos E, Schwedhelm E, Lackner KJ, Debus ES, Munzel T, Blankenberg S, Espinola-Klein C (2014) Association of multiple biomarkers and classical risk factors with early carotid atherosclerosis: results from the Gutenberg Health Study. Clin Res Cardiol Off J Ger Card Soc 103:477–485. doi:10.1007/s00392-014-0674-6
Sjöberg BG, Su J, Dahlbom I, Grönlund H, Wikström M, Hedblad B, Berglund G, de Faire U, Frostegård J (2009) Low levels of IgM antibodies against phosphorylcholine—a potential risk marker for ischemic stroke in men. Atherosclerosis 203:528–532. doi:10.1016/j.atherosclerosis.2008.07.009
Sobel M, Moreno KI, Yagi M, Kohler TR, Tang GL, Clowes AW, Zhou X-HA, Eugenio E (2013) Low levels of a natural IgM antibody are associated with vein graft stenosis and failure. J Vasc Surg 58:997–1005. doi:10.1016/j.jvs.2013.04.042
Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL (1989) Beyond Cholesterol. N Engl J Med 320:915–924. doi:10.1056/NEJM198904063201407
Su J, Georgiades A, Wu R, Thulin T, de Faire U, Frostegård J (2006) Antibodies of IgM subclass to phosphorylcholine and oxidized LDL are protective factors for atherosclerosis in patients with hypertension. Atherosclerosis 188:160–166
Virmani R, Burke AP, Farb A, Kolodgie FD (2002) Pathology of the unstable plaque. Prog Cardiovasc Dis 44:349–356
Whelton PK (1994) Epidemiology of hypertension. Lancet 344:101–106. doi:10.1016/S0140-6736(94)91285-8
Acknowledgments
We thank Nadine Wambsganss for excellent technical assistance.
Conflict of interest
Göran Conradson works for Athera.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gleissner, C.A., Erbel, C., Haeussler, J. et al. Low levels of natural IgM antibodies against phosphorylcholine are independently associated with vascular remodeling in patients with coronary artery disease. Clin Res Cardiol 104, 13–22 (2015). https://doi.org/10.1007/s00392-014-0750-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-014-0750-y