Abstract
Background and aims
In inflammatory bowel diseases iron contributes to the formation of DNA adducts through the production of hydroxyl radicals. The aim of our study was to evaluate the effects of dietary or pharmacological iron deprivation in an experimental model of colitis in the rat and its potential protective effect against DNA damage.
Methods
Colitis was induced in rats by intracolonic instillation of dinitrobenzene sulphonic acid. Rats were assigned to an iron-deprived diet or to desferrioxamine preceding the induction of colitis. The severity of colitis was assessed by the presence of bloody diarrhea, colonic macroscopic damage score, body-weight variations and the amount of DNA colonic adducts. Hepatic and colonic iron concentrations were measured.
Results
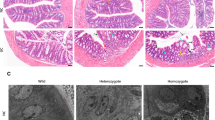
Treated rats experienced less diarrhea and did not lose weight in comparison to untreated animals. The macroscopic damage score was significantly reduced in the iron-deprived diet for the 5-week group (P=0.03). Liver and colonic iron levels were significantly more reduced in the iron-deprived groups than in the standard diet group (P<0.03 and P<0.01 after a 3- and 5-week iron-deprived diet, respectively). DNA adduct formation was significantly reduced in the groups deprived of iron for 5 weeks (P<0.001) or treated with desferrioxamine (P<0.01).
Conclusions
The degree of colitis caused by DNBS is macroscopically improved by dietary iron deprivation and to a lesser extent by pharmacological chelation; genomic damage is reduced by dietary iron deprivation or chelation, and this may have clinical implications on cancer prevention.


Similar content being viewed by others
References
Babbs CF (1992) Oxygen radicals in ulcerative colitis. Free Rad Biol Med 13:169–182
Lih-Brodi L, Powell SR, Collier KP, Reddy GM, Cerchia R, Kahn E, Weissman GS, Katz S, Floyd RA, et al (1996) Increased oxidative stress and decreased antioxidant defences in mucosa of inflammatory bowel disease. Dig Dis Sci 41:2078–2086
Sturniolo GC, Mestriner C, Lecis PE, D’Odorico A, Venturi C, Irato P, Cecchetto A, Tropea A, Longo G, et al (1998) Altered plasma and mucosal concentrations of trace elements and antioxidants in active ulcerative colitis. Scand J Gastroenterol 33:644–649
Halliwell B, Gutteridge JMC (1990) Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol 86:1–85
Eybl V, Kotyzova D, Kolek M, Katensky J, Nielsen P (2002) The influence of Deferiprone (L1) and deferoxamine on iron and essential element tissue level and parameters of oxidative status in dietary iron-loaded mice. Toxicol Lett 128:169–175
Winyard PG, Blake DR, Chirico S, Gutteridge JM, Lunec J (1987) Mechanism of exacerbation of rheumatoid synovitis by tota-dose iron infusion: in-vivo demonstration of iron-promoted oxidant stress. Lancet 1:69–72
Kawai M, Sumimoto S, Kasajima Y, Hamamoto T (1992) A case of ulcerative colitis induced by oral ferrous sulfate. Acta Paediatr Jpn 34:476–478
Erichsen K, Hausken T, Berge R (1998) Effect of oral iron therapy on antioxidant defense in active Crohn’s disease. Digestion 59:FOLM 1287
Cuzzocrea S, Mazzon E, De Sarro A, Caputi AP (2000) Role of free radicals and poly (ADP-ribose) synthethase in intestinal tight junction permeability. Mol Med 6:766–778
Robinson CE, Kottapalli V, D’Astice M, Fields JZ, Winship D, Keshavarzian A (1997) Regulation of neutrophils in ulcerative colitis by colonic factors: a possible mechanism of neutrophil activation and tissue damage. J Lab Clin Med 130:590–606
Lin M, Rippe RA, Niemela O, Brittenham G, Tsukamoto H (1997) Role of iron in NF-kappa B activation and cytokine gene expression by rat hepatic macrophages. Am J Physiol 272:G1355–64
Schmidt KN, Amstad P, Cerutti B, Baeuerle PA (1995) The role of hydrogen peroxide and superoxide as messengers in the activation of transcription factor NF-kB. Chem Biol 2:13–22
Halliwell B, Aruoma OL (1991) DNA damage by oxygen-derived species. FEBS Lett 281:9–19
Toyokuni S (2002) Iron and carcinogenesis: from Fenton reaction to target genes. Redox Rep 7:189–197
Polla BS (1999) Therapy by taking away: the case of iron. Biochem Pharmacol 57:1345–1349
Giardina PJ, Grady RW. (1995) Chelation therapy in beta-thalassemia: the benefits and limitations of desferrioxamine. Semin Hematol 32:304–312
Donfrancesco A, De Bernardi B, Carli M, Mancini A, Nigro M, De Sio L, Casale F, Bagnulo S, Helson L, et al (1995) Deferoxamine followed by cyclophosphamide, etoposide, carboplatin, thiotepa, induction regimen in advanced neuroblastoma: preliminary results. Italian Neuroblastoma Cooperative Group. Eur J Cancer 31A:612–615
Crapper MDR, Dalton AJ, Kruck TP, Bell MY, Smith WL, Kalow W (1991) Intramuscular desferrioxamine in patients with Alzheimer’s disease. Lancet 337:1304–1308
Kruck TP, Fischer EA, Mc Lachlan DR (1993) A predictor for side effects in patients with Alzheimer’s disease treated with deferoxamine mesylate. Clin Pharmacol Ther 53:30–37
Schnellmann JG, Pumford NR, Kusewitt DF, Bucci TJ, Hinson JA (1999) Deferoxamine delays the development of the hepatotoxicity of acetaminophen in mice. Toxicol Lett 106:79–88
Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL (1989) Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology 98:795–803
Fraga CG, Shigenaga MK, Park JW, Degan P, Ames BN (1990) Oxidative damage to DNA during aging: 8-hydroxy-2-deoxyguanosine in rat organ DNA and urine. Proc Natl Acad Sci USA 87:4533–4537
Helbock HJ, Beckman KB, Shigenaga MK, Walter PB, Woodall AA, Yeo HC, Ames BN (1998) DNA oxidation matters: the HPLC-electrochemical detection assay of 8-oxo-deoxyguanosine and 8-oxo-guanosine. Proc Natl Acad Sci USA 95:288–293
Reifen R, Matas Z, Zeidel L, Berkovitch Z, Bujanover Y (2000) Iron supplementation may aggravate inflammatory status of colitis in a rat model. Dig Dis Sci 45:394–397
Carrier J, Aghdassi E, Platt I, Mullen J, Allard JP (2001) Effect of oral iron supplementation on oxidative stress and colonic inflammation in rats with induced colitis. Aliment Pharmacol Ther15:1989–1999
Carrier J, Aghdassi E, Cullen J, Allord JP (2002) Iron supplementation increases disease activity and vitamin E ameliorates the effect in rats with dextran sulfate sodium-induced colitis. J Nutr 132:3146–3150
Haq RU, Werely JP, Chitambar CR (1995) Induction of apoptosis by iron deprivation in human leukemic CCRF-CEM cells. Exp Hematol 23:428–432
Kovar J, Stunz LL, Stewart BC, Kriegerbeckova K, Ashman RF, Kemp JD (1997) Direct evidence that iron deprivation induces apoptosis in murine lymphoma 38C13. Pathobiology 65:61–68
Toyokuni S (2002) Iron and carcinogenesis: from Fenton reaction to target genes. Redox Rep 7:189–197
Ablin J, Shalev O, Okon E, Karmeli F, Rachmilewitz D (1999) Deferiprone, an oral iron chelator, ameliorates experiment colitis and gastric ulceration in rats. Inflamm Bowel Dis 5:253–261
Emerit J, Pelletier S, Likforman J, Pasquier C, Thuillier A (1991) Phase II trial of copper zinc superoxide dismutase (CuZn SOD) in the treatment of Crohn’s disease. Free Radic Res Commun 12–13:563–569
Millar AD, Rampton DS, Blake DR (2000) Effects of iron and iron chelation in vitro on mucosal oxidant activity in ulcerative colitis. Aliment Pharmacol Ther 14:1163–1168
Carotenuto P, Pontesilli O, Cambier JC, Hayward AR (1986) Desferoxamine blocks IL 2 receptor expression on human T lymphocytes. J Immunol 136:2342–2347
Hoffbrand AV, Ganeshaguru K, Hooton JW, Tattersal MH (1976) Effects of iron deficiency and desferrioxamine on DNA synthesis in human cells. Br J Haematol 33:517–526
Oldemburg B, Koningsberger JC, Van Berge Henegouwen GP, Van Asbeck BS, Marks JJM (2001) Review article: iron and inflammatory bowel disease. Aliment Pharmacol Ther 15:429–438
Seril DN, Liao J, Ho KL, Warsi A, Yang CS, Yang GY (2002) Dietary iron supplementation enhances DSS-induced colitis and associated colorectal carcinoma development in mice. Dig Dis Sci 47:1266–278
Christensen DW, Kisling R, Thompson J, Kirby MA (2001) Deferoxamine toxicity in hepatoma and primary rat cortical brain cultures. Hum Exp Toxicol 20:365–372
Acknowledgements
The authors thank Mrs. M. Minotto and Mrs. C. Carlotto for their technical assistance. This work was supported in part by MIUR 60% 2000.
Author information
Authors and Affiliations
Corresponding author
Additional information
M. Barollo and R. D’Incà contributed equally to this paper
Rights and permissions
About this article
Cite this article
Barollo, M., D’Incà, R., Scarpa, M. et al. Effects of iron deprivation or chelation on DNA damage in experimental colitis. Int J Colorectal Dis 19, 461–466 (2004). https://doi.org/10.1007/s00384-004-0588-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-004-0588-2