Abstract
Purpose
Hirschsprung’s disease (HSCR) is a functional obstruction of the gastrointestinal tract due to the congenital absence of enteric ganglion cells. The proto-oncogene RET is one of the primary genes implicated in the aetiology of HSCR. We designed this study to investigate the expression of 10 RET regulatory network genes in the colons of patients with HSCR.
Methods
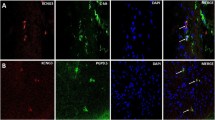
HSCR tissue specimens (n = 28) were collected at the time of pull-through surgery. qPCR analysis was applied to compare the expression levels of 10 genes in the RET regulatory network. Western blot analysis was performed to quantify the protein expression. Immunohistochemistry was performed to determine the localization of AKT1 and P38A in HSCR colon tissue.
Results
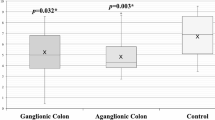
AKT1 (p = 0.015) and P38A (p = 0.039) were both significantly downregulated in the aganglionic segment compared to those in the ganglionic segment in HSCR patients (n = 28). Western blot analysis revealed the decreasing protein expression of AKT1 and P38A in the aganglionic segment compared to ganglionic segment and control colon tissues (p < 0.05). Immunohistochemistry staining revealed that both AKT1 and P38A were localized in the colonic mucosa and were significantly decreased in the aganglionic segment.
Conclusion
To our knowledge, we report for the first time the expression of RET regulatory network genes in the colons of patients with HSCR. The markedly decreased expression of AKT1 and P38A suggested a possible role in HSCR pathogenesis.




Similar content being viewed by others
References
Luzón-Toro B, Villalba-Benito L, Torroglosa A, Fernández RM, Antiñolo GBS (2019) What is new about the genetic background of Hirschsprung disease? Clin Genetics 97(1):114–124. https://doi.org/10.1111/cge.13615
Soret R, Mennetrey M, Bergeron KF, Dariel A, Neunlist M, Grunder F, Faure C, Silversides DW, Pilon N (2015) A collagen VI–dependent pathogenic mechanism for Hirschsprung’s disease. J Clin Invest 125(12):4483–4496. https://doi.org/10.1172/jci83178
Chatterjee S, Kapoor A, Akiyama JA, Auer DR, Lee D, Gabriel S, Berrios C, Pennacchio LA, Chakravarti A (2016) Enhancer variants synergistically drive dysfunction of a gene regulatory network in Hirschsprung disease. Cell 167(2):355–368. https://doi.org/10.1016/j.cell.2016.09.005(e310)
Chatterjee SAC (2019) A gene regulatory network explains RET-EDNRB epistasis in Hirschsprung disease. Hum Mol Genet 28(18):3137–3147. https://doi.org/10.1093/hmg/ddz149
Davis WJ, Lehmann PZ, Li W (2015) Nuclear PI3K signaling in cell growth and tumorigenesis. Front Cell Dev Biol. https://doi.org/10.3389/fcell.2015.00024
Hung YP, Teragawa C, Kosaisawe N, Gillies TE, Pargett M, Minguet M, Distor K, Rocha-Gregg BL, Coloff JL, Keibler MA, Stephanopoulos G, Yellen G, Brugge JS, Albeck JG (2017) Akt regulation of glycolysis mediates bioenergetic stability in epithelial cells. Elife 6:e27293. https://doi.org/10.7554/eLife.27293
Shim EJ, Bang BR, Kang SG, Ma J, Otsuka M, Kang J, Stahl M, Han J, Xiao C, Vallance BA, Kang YJ (2013) Activation of p38α in T cells regulates the intestinal host defense against attaching and effacing bacterial infections. J Immunol 191(5):2764–2770. https://doi.org/10.4049/jimmunol.1300908
Liu Y, Wang Z, Xie W, Gu Z, Xu Q, Su L (2017) Oxidative stress regulates mitogen-activated protein kinases and c-Jun activation involved in heat stress and lipopolysaccharide-induced intestinal epithelial cell apoptosis. Mol Med Rep 16(3):2579–2587. https://doi.org/10.3892/mmr.2017.6859
Kondo Y, Higa-Nakamine S, Maeda N, Toku S, Kakinohana M, Sugahara K, Kukita I, Hideyuki Y (2016) Stimulation of cell migration by flagellin through the p38 MAP kinase pathway in cultured intestinal epithelial cells. J Cell Biochem 117(1):247–258. https://doi.org/10.1002/jcb.25272
Osaki L, Gama P (2013) MAPKs and signal transduction in the control of gastrointestinal epithelial cell proliferation and differentiation. Int J Mol Sci 14(5):10143–10161. https://doi.org/10.3390/ijms140510143
Otsuka M, Kang YJ, Ren J, Jiang H, Wang Y, Omata M, Han J (2010) Distinct effects of p38α deletion in myeloid lineage and gut epithelia in mouse models of inflammatory bowel disease. Gastroenterology 138(4):1255–1265.e1259. https://doi.org/10.1053/j.gastro.2010.01.005
Alwhaibi A, Verma A, Adil MS, Somanath PR (2019) The unconventional role of Akt1 in the advanced cancers and in diabetes-promoted carcinogenesis. Pharmacol Res 145:104270. https://doi.org/10.1016/j.phrs.2019.104270
Martini M, De Santis MC, Braccini L, Gulluni F, Hirsch E (2014) PI3K/AKT signaling pathway and cancer: an updated review. Ann Med 46(6):372–383. https://doi.org/10.3109/07853890.2014.912836
Mundi PS, Sachdev J, McCourt C, Kalinsky K (2016) AKT in cancer: new molecular insights and advances in drug development. Br J Clin Pharmacol 82(4):943–956. https://doi.org/10.1111/bcp.13021
Kent LN, Ohboshi S, Soares MJ (2012) Akt1 and insulin-like growth factor 2 (Igf2) regulate placentation and fetal/postnatal development. Int J Dev Biol 56(4):255–261. https://doi.org/10.1387/ijdb.113407lk
Sharma N, Kubaczka C, Kaiser S, Nettersheim D, Mughal SS, Riesenberg S, Hölzel M, Winterhager E, Schorle H (2016) Tpbpa-Cre-mediated deletion of TFAP2C leads to deregulation of Cdkn1a, Akt1and the ERK pathway, causing placental growth arrest. Development 143(5):787–798. https://doi.org/10.1242/dev.128553
Plaks V, Berkovitz E, Vandoorne K, Berkutzki T, Damari GM, Haffner R, Dekel N, Hemmings BA, Neeman M, Harmelin A (2011) Survival and size are differentially regulated by placental and fetal PKBalpha/AKT1 in Mice1. Biol Reprod 84(3):537–545. https://doi.org/10.1095/biolreprod.110.085951
Ulici V, Hoenselaar KD, Agoston H, McErlain DD, Umoh J, Chakrabarti S, Holdsworth DW, Beier F (2009) The role of Akt1 in terminal stages of endochondral bone formation: angiogenesis and ossification. Bone 45(6):1133–11345. https://doi.org/10.1016/j.bone.2009.08.003
Yung HW, Calabrese S, Hynx D, Hemmings BA, Cetin I, Charnock-Jones DS, Burton GJ (2008) Evidence of placental translation inhibition and endoplasmic reticulum stress in the etiology of human intrauterine growth restriction. Am J Pathol 173(2):451–462. https://doi.org/10.2353/ajpath.2008.071193
Li Y, Zhou L, Lu C, Shen Q, Su Y, Zhi Z, Wu F, Zhang H, Wen Z, Chen G, Li H, Xia Y, Wang T (2018) Long non-coding RNA FAL1 functions as a ceRNA to antagonize the effect of miR-637 on the down-regulation of AKT1 in Hirschsprung's disease. Cell Prolif 51(5):e12489. https://doi.org/10.1111/cpr.12489
Yu H, Littlewood T, Bennett M (2015) Akt isoforms in vascular disease. Vasc Pharmacol 71:57–64. https://doi.org/10.1016/j.vph.2015.03.003
Lee MY, Luciano AK, Ackah E, Rodriguez-Vita J, Bancroft TA, Eichmann A, Simons M, Kyriakides TR, Morales-Ruiz M, Sessa WC (2014) Endothelial Akt1 mediates angiogenesis by phosphorylating multiple angiogenic substrates. Proc Natl Acad Sci 111(35):12865–12870. https://doi.org/10.1073/pnas.1408472111
Huang X, Liu G, Guo J, Su Z (2018) The PI3K/AKT pathway in obesity and type 2 diabetes. Int J Biol Sci 14(11):1483–1496. https://doi.org/10.7150/ijbs.27173
Youssif C, Cubillos-Rojas M, Comalada M, Llonch E, Perna C, Djouder N, Nebreda AR (2018) Myeloid p38α signaling promotes intestinal IGF-1 production and inflammation-associated tumorigenesis. EMBO Mol Med. https://doi.org/10.15252/emmm.201708403
Wakeman D, Schneider JE, Liu J, Wandu WS, Erwin CR, Guo J, Stappenbeck TS, Warner BW (2012) Deletion of p38-alpha mitogen-activated protein kinase within the intestinal epithelium promotes colon tumorigenesis. Surgery 152(2):286–293. https://doi.org/10.1016/j.surg.2012.05.009
Gupta J, del Barco BI, Igea A, Sakellariou S, Pateras IS, Gorgoulis VG, Nebreda AR (2014) Dual function of p38α MAPK in colon cancer: suppression of colitis-associated tumor initiation but requirement for cancer cell survival. Cancer Cell 25(4):484–500. https://doi.org/10.1016/j.ccr.2014.02.019
Desideri E, Vegliante R, Cardaci S, Nepravishta R, Paci M, Ciriolo MR (2014) MAPK14/p38α-dependent modulation of glucose metabolism affects ROS levels and autophagy during starvation. Autophagy 10(9):1652–1665. https://doi.org/10.4161/auto.29456
O’Donnell AM, Nakamura H, Tomuschat C, Marayati NF, Puri P (2018) Altered expression of KCNG3 and KCNG4 in Hirschsprung’s disease. Pediatr Surg Int 35(2):193–197. https://doi.org/10.1007/s00383-018-4394-2
O’Donnell A-M, Coyle D, Puri P (2016) Deficiency of platelet-derived growth factor receptor-α-positive cells in Hirschsprung's disease colon. World J Gastroenterol 22(12):3335–3340. https://doi.org/10.3748/wjg.v22.i12.3335
Tomuschat C, O'Donnell AM, Coyle D, Puri P (2019) Increased protease activated receptors in the colon of patients with Hirschsprung's disease. J Pediatr Surg. https://doi.org/10.1016/j.jpedsurg.2019.11.009
Nakamura H, O’Donnell AM, Tomuschat C, Coyle D, Puri P (2019) Altered expression of caveolin-1 in the colon of patients with Hirschsprung’s disease. Pediatr Surg Int 35(9):929–934. https://doi.org/10.1007/s00383-019-04505-1
Gunadi KAS, Liana E, Fauzi AR, Sirait DN, Afandy D, Kencana SMS, Purnomo E, Iskandar K, Makhmudi A (2019) Aberrant UBR4 expressions in Hirschsprung disease patients. BMC Pediatr. https://doi.org/10.1186/s12887-019-1879-7
Gunadi BNYP, Kalim AS, Santiko W, Musthofa FD, Iskandar K, Makhmudi A (2019) Aberrant expressions of miRNA-206 target, FN1, in multifactorial Hirschsprung disease. Orphanet J Rare Dis. https://doi.org/10.1186/s13023-018-0973-5
Funding
This study was funded by the National Natural Science Foundation of China (Grant number: 81771620) to QJ.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors disclose no conflicts.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Ethical Committee of the Capital Institute of Pediatrics and with the 1964 Helsinki Declaration and its later amendments.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wu, L., Xiao, P., Li, Q. et al. Altered expression of AKT1 and P38A in the colons of patients with Hirschsprung’s disease. Pediatr Surg Int 36, 719–725 (2020). https://doi.org/10.1007/s00383-020-04653-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-020-04653-9