Abstract
Purpose
The Wnt/β-catenin pathway is known to be crucial for the regulation of embryogenesis and cell differentiation, and its constitutive activation is associated with a wide range of malignancies. There are two major principles for an activated Wnt/β-catenin pathway. The first is caused by the failure of the destruction complex, mainly due to the decreased expression of the tumor suppressor gene adenomatous polyposis coli (APC); the second is the mutation of the β-catenin (CTNNB1) protein itself. Wilms tumors (WTs) are also thought to be malignancies with a high rate of Wnt/β-catenin pathway activation. The aim of this study was to analyze a large cohort of WT for activated Wnt/β-catenin pathway.
Methods
The transcription of axis inhibition protein 2 (AXIN2) and APC was analyzed by real-time PCR. Expression was compared with those in healthy renal tissues as a control. Methylation status of the APC promoter was measured by pyrosequencing and correlated with APC expression. Finally, the mutations of CTNNB1 itself were detected by Sanger sequencing.
Results
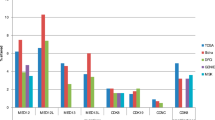
The analysis was done in a cohort of 103 WTs, treated in our institution. There was a significant overexpression of AXIN2 in WTs (P < 0.0001), with 33 (32 %) tumors showing higher expression (median + 3× SD) than normal kidney tissue. In contrast, the expression of APC as well as its promoter methylation did not differ from control (P = 0.78; P = 0.82). Finally, there were only seven (6.8 %) mutations detectable in CTNNB1, and five out of seven were seen in WTs with AXIN2 overexpression.
Conclusion
The finding that AXIN2, one of the major Wnt target genes, is overexpressed in our cohort of WTs, is indicative for the activation of the Wnt/β-catenin pathway. However, neither the alteration of APC nor frequent CTNNB1 mutations were seen in our analyses. Therefore, other mechanisms might be responsible for the common activation of the Wnt/β-catenin pathway.


Similar content being viewed by others
References
Breslow N et al (1988) Age distribution of Wilms’ tumor: report from the National Wilms’ Tumor Study. Cancer Res 48(6):1653–1657
Breslow N et al (1993) Epidemiology of Wilms tumor. Med Pediatr Oncol 21(3):172–181
Metzger ML, Dome JS (2005) Current therapy for Wilms’ tumor. Oncologist 10(10):815–826
Li CM et al (2002) Gene expression in Wilms’ tumor mimics the earliest committed stage in the metanephric mesenchymal-epithelial transition. Am J Pathol 160(6):2181–2190
Scott RH et al (2012) Stratification of Wilms tumor by genetic and epigenetic analysis. Oncotarget 3(3):327–335
Rakheja D et al (2014) Somatic mutations in DROSHA and DICER1 impair microRNA biogenesis through distinct mechanisms in Wilms tumours. Nat Commun 2:4802
Ruteshouser EC, Robinson SM, Huff V (2008) Wilms tumor genetics: mutations in WT1, WTX, and CTNNB1 account for only about one-third of tumors. Genes Chromosomes Cancer 47(6):461–470
Wittmann S et al (2008) New prognostic markers revealed by evaluation of genes correlated with clinical parameters in Wilms tumors. Genes Chromosomes Cancer 47(5):386–395
Astuti D et al (2001) Germline SDHD mutation in familial phaeochromocytoma. Lancet 357(9263):1181–1182
Williams RD et al (2010) Subtype-specific FBXW7 mutation and MYCN copy number gain in Wilms’ tumor. Clin Cancer Res 16(7):2036–2045
Walz AL et al (2015) Recurrent DGCR8, DROSHA, and SIX homeodomain mutations in favorable histology Wilms tumors. Cancer Cell 27(2):286–297
Torrezan GT et al (2014) Recurrent somatic mutation in DROSHA induces microRNA profile changes in Wilms tumour. Nat Commun 5:4039
Wegert J et al (2015) Mutations in the SIX1/2 pathway and the DROSHA/DGCR8 miRNA microprocessor complex underlie high-risk blastemal type Wilms tumors. Cancer Cell 27(2):298–311
Bjornsson HT et al (2007) Epigenetic specificity of loss of imprinting of the IGF2 gene in Wilms tumors. J Natl Cancer Inst 99(16):1270–1273
Hubertus J et al (2011) Altered expression of imprinted genes in Wilms tumors. Oncol Rep 25(3):817–823
Zitzmann F et al (2014) Frequent hypermethylation of a CTCF binding site influences Wilms tumor 1 expression in Wilms tumors. Oncol Rep 31(4):1871–1876
Hubertus J et al (2013) Selective methylation of CpGs at regulatory binding sites controls NNAT expression in Wilms tumors. PLoS One 8(6):e67605
Cardoso LC et al (2013) WT1, WTX and CTNNB1 mutation analysis in 43 patients with sporadic Wilms’ tumor. Oncol Rep 29(1):315–320
Perotti D et al (2013) Is Wilms tumor a candidate neoplasia for treatment with WNT/beta-catenin pathway modulators?—a report from the renal tumors biology-driven drug development workshop. Mol Cancer Ther 12(12):2619–2627
Stamos JL, Weis WI (2013) The beta-catenin destruction complex. Cold Spring Harb Perspect Biol 5(1):a007898
Tai D et al (2015) Targeting the WNT signaling pathway in cancer therapeutics. Oncologist 20(10):1189–1198
Valenta T, Lukas J, Korinek V (2003) HMG box transcription factor TCF-4’s interaction with CtBP1 controls the expression of the Wnt target Axin2/Conductin in human embryonic kidney cells. Nucleic Acids Res 31(9):2369–2380
He TC et al (1998) Identification of c-MYC as a target of the APC pathway. Science 281(5382):1509–1512
de Kraker J et al (2004) Reduction of postoperative chemotherapy in children with stage I intermediate-risk and anaplastic Wilms’ tumour (SIOP 93-01 trial): a randomised controlled trial. Lancet 364(9441):1229–1235
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29(9):e45
Koch A et al (1999) Childhood hepatoblastomas frequently carry a mutated degradation targeting box of the beta-catenin gene. Cancer Res 59(2):269–273
Esteller M et al (2000) Analysis of adenomatous polyposis coli promoter hypermethylation in human cancer. Cancer Res 60(16):4366–4371
Sparks AB et al (1998) Mutational analysis of the APC/beta-catenin/Tcf pathway in colorectal cancer. Cancer Res 58(6):1130–1134
Park WS et al (1999) Frequent somatic mutations of the beta-catenin gene in intestinal-type gastric cancer. Cancer Res 59(17):4257–4260
Polakis P (2012) Wnt signaling in cancer. Cold Spring Harb Perspect Biol 4(5):a008052. doi:10.1101/cshperspect.a008052
Aiden AP et al (2010) Wilms tumor chromatin profiles highlight stem cell properties and a renal developmental network. Cell Stem Cell 6(6):591–602
Kispert A, Vainio S, McMahon AP (1998) Wnt-4 is a mesenchymal signal for epithelial transformation of metanephric mesenchyme in the developing kidney. Development 125(21):4225–4234
Koesters R et al (1999) Mutational activation of the beta-catenin proto-oncogene is a common event in the development of Wilms’ tumors. Cancer Res 59(16):3880–3882
Bernkopf DB, Hadjihannas MV, Behrens J (2015) Negative-feedback regulation of the Wnt pathway by conductin/axin2 involves insensitivity to upstream signalling. J Cell Sci 128(1):33–39
Amit S et al (2002) Axin-mediated CKI phosphorylation of beta-catenin at Ser 45: a molecular switch for the Wnt pathway. Genes Dev 16(9):1066–1076
Barros BD et al (2012) Mutational spectrum of WTX, WT1, CTNNB1, APC and PLCG2 genes in Wilms tumor defined by massive parallel resequencing. BMC Proc 6(Suppl 6):P52. doi:10.1186/1753-6561-6-S6-P52
Wissmann C et al (2003) WIF1, a component of the Wnt pathway, is down-regulated in prostate, breast, lung, and bladder cancer. J Pathol 201(2):204–212
Gumz ML et al (2007) Secreted frizzled-related protein 1 loss contributes to tumor phenotype of clear cell renal cell carcinoma. Clin Cancer Res 13(16):4740–4749
Zirn B et al (2006) Target genes of the WNT/beta-catenin pathway in Wilms tumors. Genes Chromosomes Cancer 45(6):565–574
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Schweigert, A., Fischer, C., Mayr, D. et al. Activation of the Wnt/β-catenin pathway is common in wilms tumor, but rarely through β-catenin mutation and APC promoter methylation. Pediatr Surg Int 32, 1141–1146 (2016). https://doi.org/10.1007/s00383-016-3970-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-016-3970-6