Abstract
Purpose
The diagnosis of Hirschsprung’s disease (HD) was revolutionized by the introduction of rectal suction biopsy (RSB), allowing specimens to be taken without general anesthesia on the ward or as an out-patient procedure. However, insufficient tissue samples are not uncommon, and subsequently histopathologists often remain reluctant to confirm the presence or absence of enteric ganglion cells merely on the basis of submucosal RSBs. The aim of this study was to evaluate the current usage of RSB in the diagnostic work-up of HD based on an international survey.
Methods
A 15-item questionnaire was distributed among participants and faculty members at the 21st International Meeting of the Pediatric Colorectal Society.
Results
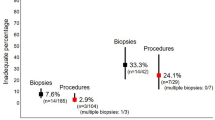
Eighty-seven pediatric surgeons from 30 countries completed the anonymous survey (response rate 70.2 %), grouped into 68 (78.2 %) staff surgeons and 19 (21.8 %) trainees, with a median work experience of 18 years (range 2–45 years). Of these, 74 (85.1 %) use RSB in the diagnostic work-up of patients with suspected HD, whereas 13 (14.9 %) prefer open full-thickness biopsy under general anesthesia. In total, 47 (63.5 %) respondents perform ≥20 RSBs (range 3–100 RSBs) per year. Five different RSB instruments were reported, the most common ones being rbi2 (65.0 %), Solo-RBT (15.0 %) and multipurpose suction biopsy kit (8.3 %). Only 22 (29.7 %) of the respondents use a defined negative suction pressure, with a median of 10 mL air (range 6–25 mL air). The most proximal reported biopsy site was located at a median of 2 cm (range 1–15 cm) above the pectinate line and a median of 2 (range 1–5) specimens are routinely taken, mainly from the posterior rectal wall. Insufficient tissue samples with need for repeat RSB were encountered in a median of 10 % (range 0–40 %). Most frequently used staining methods for rectal biopsies are hematoxylin/eosin (75.9 %), acetylcholinesterase (73.6 %), and calretinin (33.3 %). Overall, 36 (48.6 %) respondents had experienced RSB-related complications, including self-limiting rectal blood loss (n = 28), persistent rectal bleeding requiring blood transfusion (n = 9) and rectal perforation requiring surgical intervention (n = 7).
Conclusions
Although RSB is considered to be today’s gold standard for the diagnosis of HD, many aspects of its current usage are lacking consensus. Therefore, a prospective multi-center study or larger global audit appears warranted to identify if the present survey reflects common surgical practice and to establish universal standards for RSB.

Similar content being viewed by others
References
Kapur RP (1999) Hirschsprung disease and other enteric dysganglionoses. Crit Rev Clin Lab Sci 36(3):225–273
Monforte-Muñoz H, Gonzalez-Gomez I, Rowland JM et al (1998) Increased submucosal nerve trunk caliber in aganglionosis: a “positive” and objective finding in suction biopsies and segmental resections in Hirschsprung’s disease. Arch Pathol Lab Med 122(8):721–725
Dobbins WO 3rd, Bill AH Jr (1965) Diagnosis of Hirschsprung’s disease excluded by rectal suction biopsy. N Engl J Med 272(13):990–993
Noblett HR (1969) A rectal suction biopsy tube for use in the diagnosis of Hirschsprung’s disease. J Pediatr Surg 4(4):406–409
Martucciello G, Pini Prato A, Puri P et al (2005) Controversies concerning diagnostic guidelines for anomalies of the enteric nervous system: a report from the fourth International Symposium on Hirschsprung’s disease and related neurocristopathies. J Pediatr Surg 40(10):1527–1531
Qualman SJ, Jaffe R, Bove KE et al (1999) Diagnosis of Hirschsprung disease using the rectal biopsy: multi-institutional survey. Pediatr Dev Pathol 2(6):588–596
Knowles CH, De Giorgio R, Kapur RP et al (2010) The London classification of gastrointestinal neuromuscular pathology: report on behalf of the Gastro 2009 International Working Group. Gut 59(7):882–887
Hall NJ, Kufeji D, Keshtgar A (2009) Out with the old and in with the new: a comparison of rectal suction biopsies with traditional and modern biopsy forceps. J Pediatr Surg 44(2):395–398
Dahshan A (2014) Serious rectal bleeding complicating suction rectal biopsy in a child. W V Med J 110(2):34–35
Pini-Prato A, Carlini C, Pesce F et al (2011) Massive bleeding after rectal suction biopsy: uncommon and unexpected delayed onset. World J Pediatr 7(1):83–85
Rees BI, Azmy A, Nigam M et al (1983) Complications of rectal suction biopsy. J Pediatr Surg 18(3):273–275
Friedmacher F, Puri P (2015) Rectal suction biopsy for the diagnosis of Hirschsprung’s disease: a systematic review of diagnostic accuracy and complications. Pediatr Surg Int 31(9):821–830
Kurer MH, Lawson JO, Pambakian H (1986) Suction biopsy in Hirschsprung’s disease. Arch Dis Child 61(1):83–84
Yunis EJ, Dibbins AW, Sherman FE (1976) Rectal suction biopsy in the diagnosis of Hirschsprung disease in infants. Arch Pathol Lab Med 100(6):329–333
Lake BD, Puri P, Nixon HH et al (1978) Hirschsprung’s disease: an appraisal of histochemically demonstrated acetylcholinesterase activity in suction rectal biopsy specimens as an aid to diagnosis. Arch Pathol Lab Med 102(5):244–247
Aldridge RT, Campbell PE (1968) Ganglion cell distribution in the normal rectum and anal canal. A basis for the diagnosis of Hirschsprung’s disease by anorectal biopsy. J Pediatr Surg 3(4):475–490
Venugopal S, Mancer K, Shandling B (1981) The validity of rectal biopsy in relation to morphology and distribution of ganglion cells. J Pediatr Surg 16(4):433–437
Kapur RR (2009) Practical pathology and genetics of Hirschsprung’s disease. Semin Pediatr Surg 18(4):212–223
Ohi RJ, Tseng SW, Kamiyama T et al (1990) Two-point rectal mucosal biopsy for selection of surgical treatment of Hirschsprung’s disease. J Pediatr Surg 25(5):527–530
Campbell PE, Noblett HR (1969) Experience with rectal suction biopsy in the diagnosis of Hirschsprung’s disease. J Pediatr Surg 4(4):410–415
Alizai NK, Batcup G, Dixon MF et al (1998) Rectal biopsy for Hirschsprung’s disease: what is the optimum method? Pediatr Surg Int 13(2–3):121–124
Athow AC, Filipe MI, Drake DP (1990) Problems and advantages of acetylcholinesterase histochemistry of rectal suction biopsies in the diagnosis of Hirschsprung’s disease. J Pediatr Surg 25(5):520–526
Kobayashi H, Li Z, Yamataka A et al (2002) Rectal biopsy: what is the optimal procedure? Pediatr Surg Int 18(8):753–756
Ali AE, Morecroft JA, Bowen JC et al (2006) Wall or machine suction rectal biopsy for Hirschsprung’s disease: a simple modified technique can improve the adequacy of biopsy. Pediatr Surg Int 22(8):681–682
Croffie JM, Davis MM, Faught PR et al (2007) At what age is a suction rectal biopsy less likely to provide adequate tissue for identification of ganglion cells? J Pediatr Gastroenterol Nutr 44(2):198–202
Muise ED, Hardee S, Morotti RA et al (2016) A comparison of suction and full-thickness rectal biopsy in children. J Surg Res 201(1):149–155
Martucciello G (2008) Hirschsprung’s disease, one of the most difficult diagnoses in pediatric surgery: a review of the problems from clinical practice to the bench. Eur J Pediatr Surg 18(3):140–149
Barshack I, Fridman E, Goldberg I et al (2004) The loss of calretinin expression indicates aganglionosis in Hirschsprung’s disease. J Clin Pathol 57(7):712–716
Meier-Ruge W, Lutterbeck PM, Herzog B et al (1972) Acetylcholinesterase activity in suction biopsies of the rectum in the diagnosis of Hirschsprung’s disease. J Pediatr Surg 7(1):11–17
de Lorijn F, Kremer LC, Reitsma JB et al (2006) Diagnostic tests in Hirschsprung disease: a systematic review. J Pediatr Gastroenterol Nutr 42(5):496–505
Meinds RJ, Kuiper GA, Parry K et al (2015) Infant’s age influences the accuracy of rectal suction biopsies for diagnosing of Hirschsprung’s disease. Clin Gastroenterol Hepatol 13(10):1801–1807
Brady AC, Saito JM, Lukas K et al (2016) Suction rectal biopsy yields adequate tissue in children. J Pediatr Surg 51(6):966–969
de Brito IA, Maksoud JG (1987) Evolution with age of the acetylcholinesterase activity in rectal suction biopsy in Hirschsprung’s disease. J Pediatr Surg 22(5):425–430
Jani BR, Brereton RJ, Dillon MJ (1989) Peripheral limb gangrene following rectal biopsy. Treatment with prostacyclin and exchange transfusion. Clin Pediatr (Phila) 28(12):585–588
Ghosh A, Griffiths DM (1998) Rectal biopsy in the investigation of constipation. Arch Dis Child 79(3):266–268
Lewis NA, Levitt MC, Zallen GS et al (2003) Diagnosing Hirschsprung’s disease: increasing the odds of a positive rectal biopsy result. J Pediatr Surg 38(3):412–416
Simpson BB, Ryan DP, Schnitzer JJ et al (1996) Surgical evaluation and management of refractory constipation in older children. J Pediatr Surg 31(8):1040–1042
Wheatley MJ, Wesley JR, Coran AG et al (1990) Hirschsprung’s disease in adolescents and adults. Dis Colon Rectum 33(7):622–629
Keyzer-Dekker CM, Sloots CE, Schokker-van Linschoten IK et al (2016) Effectiveness of rectal suction biopsy in diagnosing Hirschsprung disease. Eur J Pediatr Surg 26(1):100–105
Pini-Prato A, Martucciello G, Jasonni V (2006) Rectal suction biopsy in the diagnosis of intestinal dysganglionoses: 5-year experience with Solo-RBT in 389 patients. J Pediatr Surg 41(6):1043–1048
Goto S, Ikeda K, Toyohara T (1984) Histochemical confirmation of the acetylcholinesterase-activity in rectal suction biopsy from neonates with Hirschsprung’s disease. Z Kinderchir 39(4):246–249
Acknowledgments
The authors thank all the attendees and faculty members of the 21st International Meeting of the Pediatric Colorectal Society that participated in this survey.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Rights and permissions
About this article
Cite this article
Friedmacher, F., Puri, P. Current practice patterns of rectal suction biopsy in the diagnostic work-up of Hirschsprung’s disease: results from an international survey. Pediatr Surg Int 32, 717–722 (2016). https://doi.org/10.1007/s00383-016-3907-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00383-016-3907-0