Abstract
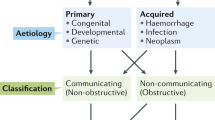
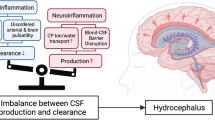
Reparative inflammation is an important protective response that eliminates foreign organisms, damaged cells, and physical irritants. However, inappropriately triggered or sustained inflammation can respectively initiate, propagate, or prolong disease. Post-hemorrhagic (PHH) and post-infectious hydrocephalus (PIH) are the most common forms of hydrocephalus worldwide. They are treated using neurosurgical cerebrospinal fluid (CSF) diversion techniques with high complication and failure rates. Despite their distinct etiologies, clinical studies in human patients have shown PHH and PIH share similar CSF cytokine and immune cell profiles. Here, in light of recent work in model systems, we discuss the concept of “inflammatory hydrocephalus” to emphasize potential shared mechanisms and potential therapeutic vulnerabilities of these disorders. We propose that this change of emphasis could shift our thinking of PHH and PIH from a framework of life-long neurosurgical disorders to that of preventable conditions amenable to immunomodulation.
Similar content being viewed by others
Abbreviations
- PHH:
-
Post-hemorrhagic hydrocephalus
- PIH:
-
Post-infectious hydrocephalus
- TLR:
-
Toll-like receptor
- CSF:
-
Cerebrospinal fluid
- ChP:
-
Choroid plexus epithelium
- CPC:
-
Choroid plexus cauterization
- NKCC1:
-
Na-K-Cl cotransporter 1
- SPAK:
-
STE20/SPS1-related, proline-alanine-rich kinase
- IVH:
-
Intraventricular hemorrhage
References
Brinker T, Stopa E, Morrison J, Klinge P (2014) A new look at cerebrospinal fluid circulation. Fluids Barriers CNS 11:10
Benveniste H, Lee H, Volkow ND (2017) The glymphatic pathway: waste removal from the CNS via cerebrospinal fluid transport. Neuroscientist 23(5):454–465
Furey CG, Choi J, Jin SC et al (2018) De novo mutation in genes regulating neural stem cell fate in human congenital hydrocephalus. Neuron 99(2):302–14.e4
Kahle KT, Kulkarni AV, Limbrick DD Jr, Warf BC (2016) Hydrocephalus in children. Lancet 387(10020):788–799
Karimy JK, Duran D, Hu JK et al (2016) Cerebrospinal fluid hypersecretion in pediatric hydrocephalus. Neurosurg Focus 41(5):E10
Karimy JK, Zhang J, Kurland DB et al (2017) Inflammation-dependent cerebrospinal fluid hypersecretion by the choroid plexus epithelium in posthemorrhagic hydrocephalus. Nat Med
Dewan MC, Rattani A, Mekary R et al (2018) Global hydrocephalus epidemiology and incidence: systematic review and meta-analysis. J Neurosurg 1–15
Cherian S, Whitelaw A, Thoresen M, Love S (2004) The pathogenesis of neonatal post-hemorrhagic hydrocephalus. Brain Pathol 14(3):305–311
Strahle J, Garton HJ, Maher CO, Muraszko KM, Keep RF, Xi G (2012) Mechanisms of hydrocephalus after neonatal and adult intraventricular hemorrhage. Transl Stroke Res 3(Suppl 1):25–38
Tully HM, Wenger TL, Kukull WA, Doherty D, Dobyns WB (2016) Anatomical configurations associated with posthemorrhagic hydrocephalus among premature infants with intraventricular hemorrhage. Neurosurg Focus 41(5):E5
Visagan R, Livermore LJ, Kelly D, Magdum S (2017) Subclinical meningoventriculitis as a cause of obstructive hydrocephalus. BMJ Case Rep 2017
Klebe D, McBride D, Krafft PR, Flores JJ, Tang J, Zhang JH (2020) Posthemorrhagic hydrocephalus development after germinal matrix hemorrhage: established mechanisms and proposed pathways. J Neurosci Res 98(1):105–120
Damkier HH, Brown PD, Praetorius J (2013) Cerebrospinal fluid secretion by the choroid plexus. Physiol Rev 93(4):1847–1892
Isaacs AM, Riva-Cambrin J, Yavin D et al (2018) Age-specific global epidemiology of hydrocephalus: Systematic review, metanalysis and global birth surveillance. PLoS One 13(10):e0204926
Warf BC (2010) East African Neurosurgical Research C. Pediatric hydrocephalus in East Africa: prevalence, causes, treatments, and strategies for the future. World Neurosurg 73(4):296–300
Muir RT, Wang S, Warf BC (2016) Global surgery for pediatric hydrocephalus in the developing world: a review of the history, challenges, and future directions. Neurosurg Focus 41(5):E11
Li L, Padhi A, Ranjeva SL et al (2011) Association of bacteria with hydrocephalus in Ugandan infants. J Neurosurg Pediatr 7(1):73–87
Schiff SJ, Ranjeva SL, Sauer TD, Warf BC (2012) Rainfall drives hydrocephalus in East Africa. J Neurosurg Pediatr 10(3):161–167
Aziz IA (1976) Hydrocephalus in the sudan. J R Coll Surg Edinb 21(4):222–224
van der Linden V, de Lima Petribu NC, Pessoa A et al (2018) Association of severe hydrocephalus with congenital Zika syndrome. JAMA Neurol
Kamat AS, Gretschel A, Vlok AJ, Solomons R (2018) CSF protein concentration associated with ventriculoperitoneal shunt obstruction in tuberculous meningitis. Int J Tuberc Lung Dis 22(7):788–792
Aranha A, Choudhary A, Bhaskar S, Gupta LN (2018) A randomized study comparing endoscopic third ventriculostomy versus ventriculoperitoneal shunt in the management of hydrocephalus due to tuberculous meningitis. Asian J Neurosurg 13(4):1140–1147
Rajshekhar V (2009) Management of hydrocephalus in patients with tuberculous meningitis. Neurol India 57(4):368–374
Li K, Tang H, Yang Y et al (2017) Clinical features, long-term clinical outcomes, and prognostic factors of tuberculous meningitis in West China: a multivariate analysis of 154 adults. Expert Rev Anti Infect Ther 15(6):629–635
Lee LV (2000) Neurotuberculosis among Filipino children: an 11 years experience at the Philippine Children’s Medical Center. Brain Dev 22(8):469–474
Kulkarni AV, Schiff SJ, Mbabazi-Kabachelor E et al (2017) Endoscopic treatment versus shunting for infant hydrocephalus in Uganda. N Engl J Med 377(25):2456–2464
Thigpen MC, Whitney CG, Messonnier NE et al (2011) Bacterial meningitis in the United States, 1998–2007. N Engl J Med 364(21):2016–2025
Pyrgos V, Seitz AE, Steiner CA, Prevots DR, Williamson PR (2013) Epidemiology of cryptococcal meningitis in the US: 1997–2009. PLoS One 8(2):e56269
Liu J, Chen ZL, Li M et al (2018) Ventriculoperitoneal shunts in non-HIV cryptococcal meningitis. BMC Neurol 18(1):58
Warf BC, Dagi AR, Kaaya BN, Schiff SJ (2011) Five-year survival and outcome of treatment for postinfectious hydrocephalus in Ugandan infants. J Neurosurg Pediatr 8(5):502–508
Chen Q, Feng Z, Tan Q et al (2017) Post-hemorrhagic hydrocephalus: Recent advances and new therapeutic insights. J Neurol Sci 375:220–230
Tsitouras V, Sgouros S (2011) Infantile posthemorrhagic hydrocephalus. Childs Nerv Syst 27(10):1595–1608
Murphy BP, Inder TE, Rooks V et al (2002) Posthaemorrhagic ventricular dilatation in the premature infant: natural history and predictors of outcome. Arch Dis Child Fetal Neonatal Ed 87(1):F37-41
Bir SC, Patra DP, Maiti TK et al (2016) Epidemiology of adult-onset hydrocephalus: institutional experience with 2001 patients. Neurosurg Focus 41(3):E5
Chahlavi A, El-Babaa SK, Luciano MG (2001) Adult-onset hydrocephalus. Neurosurg Clin N Am 12(4):753–760, ix
Cioca A, Gheban D, Perju-Dumbrava D, Chiroban O, Mera M (2014) Sudden death from ruptured choroid plexus arteriovenous malformation. Am J Forensic Med Pathol 35(2):100–102
Warf BC (2005) Comparison of endoscopic third ventriculostomy alone and combined with choroid plexus cauterization in infants younger than 1 year of age: a prospective study in 550 African children. J Neurosurg 103(6 Suppl):475–481
Warf BC (2005) Hydrocephalus in Uganda: the predominance of infectious origin and primary management with endoscopic third ventriculostomy. J Neurosurg 102(1 Suppl):1–15
Stagno V, Navarrete EA, Mirone G, Esposito F (2013) Management of hydrocephalus around the world. World Neurosurg 79(2 Suppl):S23.e17–20
Kulkarni AV (2016) First treatment in infants with hydrocephalus: the case for shunt. Neurosurgery 63(Suppl 1):73–77
Kulkarni AV, Drake JM, Kestle JR, Mallucci CL, Sgouros S, Constantini S (2010) Endoscopic third ventriculostomy vs cerebrospinal fluid shunt in the treatment of hydrocephalus in children: a propensity score-adjusted analysis. Neurosurgery 67(3):588–593
Baird LC (2016) First treatment in infants with hydrocephalus: the case for endoscopic third ventriculostomy/choroid plexus cauterization. Neurosurgery 63(Suppl 1):78–82
Kulkarni AV, Riva-Cambrin J, Butler J et al (2013) Outcomes of CSF shunting in children: comparison of Hydrocephalus Clinical Research Network cohort with historical controls: clinical article. J Neurosurg Pediatr 12(4):334–338
Anderson IA, Saukila LF, Robins JMW et al (2018) Factors associated with 30-day ventriculoperitoneal shunt failure in pediatric and adult patients. J Neurosurg 130(1):145–153
Stone JJ, Walker CT, Jacobson M, Phillips V, Silberstein HJ (2013) Revision rate of pediatric ventriculoperitoneal shunts after 15 years. J Neurosurg Pediatr 11(1):15–19
Drake JM, Kulkarni AV, Kestle J (2009) Endoscopic third ventriculostomy versus ventriculoperitoneal shunt in pediatric patients: a decision analysis. Childs Nerv Syst 25(4):467–472
Kulkarni AV, Drake JM, Mallucci CL, Sgouros S, Roth J, Constantini S (2009) Endoscopic third ventriculostomy in the treatment of childhood hydrocephalus. J Pediatr 155(2):254–9.e1
Pindrik J, Jallo GI, Ahn ES (2013) Complications and subsequent removal of retained shunt hardware after endoscopic third ventriculostomy: case series. J Neurosurg Pediatr 11(6):722–726
Limbrick DD Jr, Baird LC, Klimo P Jr, Riva-Cambrin J, Flannery AM (2014) Pediatric hydrocephalus: systematic literature review and evidence-based guidelines. Part 4: cerebrospinal fluid shunt or endoscopic third ventriculostomy for the treatment of hydrocephalus in children. J Neurosurg Pediatr 14(Suppl 1):30–4
Kulkarni AV, Riva-Cambrin J, Browd SR et al (2014) Endoscopic third ventriculostomy and choroid plexus cauterization in infants with hydrocephalus: a retrospective Hydrocephalus Clinical Research Network study. J Neurosurg Pediatr 14(3):224–229
Marques F, Sousa JC, Brito MA et al (2017) The choroid plexus in health and in disease: dialogues into and out of the brain. Neurobiol Dis 107:32–40
Tirado-Caballero J, Rivero-Garvia M, Arteaga-Romero F, Herreria-Franco J, Lozano-Gonzalez A, Marquez-Rivas J (2020) Neuroendoscopic lavage for the management of posthemorrhagic hydrocephalus in preterm infants: safety, effectivity, and lessons learned. J Neurosurg Pediatr 1–10
Schulz M, Buhrer C, Pohl-Schickinger A, Haberl H, Thomale UW (2014) Neuroendoscopic lavage for the treatment of intraventricular hemorrhage and hydrocephalus in neonates. J Neurosurg Pediatr 13(6):626–635
Qin G, Liang Y, Xu K et al (2020) Neuroendoscopic lavage for ventriculitis: Case report and literature review. Neurochirurgie 66(2):127–132
Larroche JC (1972) Post-haemorrhagic hydrocephalus in infancy. Anatomical study. Biol Neonate 20(3):287–299
Omar AT II, Bagnas MAC, Del Rosario-Blasco KAR, Diestro JDB, Khu KJO (2018) Shunt surgery for neurocutaneous melanosis with hydrocephalus: case report and review of the literature. World Neurosurg 120:583–589
Whitelaw A (2001) Intraventricular haemorrhage and posthaemorrhagic hydrocephalus: pathogenesis, prevention and future interventions. Semin Neonatol 6(2):135–146
Lategan B, Chodirker BN, Del Bigio MR (2010) Fetal hydrocephalus caused by cryptic intraventricular hemorrhage. Brain Pathol 20(2):391–398
Hill A, Shackelford GD, Volpe JJ (1984) A potential mechanism of pathogenesis for early posthemorrhagic hydrocephalus in the premature newborn. Pediatrics 73(1):19–21
Gram M, Sveinsdottir S, Cinthio M et al (2014) Extracellular hemoglobin - mediator of inflammation and cell death in the choroid plexus following preterm intraventricular hemorrhage. J Neuroinflammation 11:200
Gram M, Sveinsdottir S, Ruscher K et al (2013) Hemoglobin induces inflammation after preterm intraventricular hemorrhage by methemoglobin formation. J Neuroinflammation 10:100
Simard PF, Tosun C, Melnichenko L, Ivanova S, Gerzanich V, Simard JM (2011) Inflammation of the choroid plexus and ependymal layer of the ventricle following intraventricular hemorrhage. Transl Stroke Res 2(2):227–231
Barichello T, Fagundes GD, Generoso JS, Elias SG, Simoes LR, Teixeira AL (2013) Pathophysiology of neonatal acute bacterial meningitis. J Med Microbiol 62(Pt 12):1781–1789
Bateman GA, Brown KM (2012) The measurement of CSF flow through the aqueduct in normal and hydrocephalic children: from where does it come, to where does it go? Child’s nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery 28(1):55–63
Oi S, Di Rocco C (2006) Proposal of “evolution theory in cerebrospinal fluid dynamics” and minor pathway hydrocephalus in developing immature brain. Child’s nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery 22(7):662–669
Oreskovic D, Rados M, Klarica M (2017) Role of choroid plexus in cerebrospinal fluid hydrodynamics. Neuroscience 354:69–87
Miyajima M, Arai H (2015) Evaluation of the production and absorption of cerebrospinal fluid. Neurol Med Chir 55(8):647–656
Lohrberg M, Wilting J (2016) The lymphatic vascular system of the mouse head. Cell Tissue Res 366(3):667–677
Brinker T, Stopa E, Morrison J, Klinge P (2014) A new look at cerebrospinal fluid circulation. Fluids Barriers CNS 11:10
Keep RF, Jones HC (1990) A morphometric study on the development of the lateral ventricle choroid plexus, choroid plexus capillaries and ventricular ependyma in the rat. Brain Res Dev Brain Res 56(1):47–53
Pellegrini L, Bonfio C, Chadwick J, Begum F, Skehel M, Lancaster MA (2020) Human CNS barrier-forming organoids with cerebrospinal fluid production. Science 369(6500)
Bothwell SW, Janigro D, Patabendige A (2019) Cerebrospinal fluid dynamics and intracranial pressure elevation in neurological diseases. Fluids Barriers CNS 16(1):9
Buhrley LE, Reed DJ (1972) The effect of furosemide on sodium-22 uptake into cerebrospinal fluid and brain. Exp Brain Res 14(5):503–510
Stodberg T, Magnusson M, Lesko N et al (2020) SLC12A2 mutations cause NKCC1 deficiency with encephalopathy and impaired secretory epithelia. Neurol Genet 6(4):e478
Steffensen AB, Oernbo EK, Stoica A et al (2018) Cotransporter-mediated water transport underlying cerebrospinal fluid formation. Nat Commun 9(1):2167
Gregoriades JMC, Madaris A, Alvarez FJ, Alvarez-Leefmans FJ (2019) Genetic and pharmacological inactivation of apical Na(+)-K(+)-2Cl(-) cotransporter 1 in choroid plexus epithelial cells reveals the physiological function of the cotransporter. Am J Physiol Cell Physiol 316(4):C525–C544
Strominger I, Elyahu Y, Berner O et al (2018) The choroid plexus functions as a niche for T-cell stimulation within the central nervous system. Front Immunol 9:1066
Engelhardt B, Vajkoczy P, Weller RO (2017) The movers and shapers in immune privilege of the CNS. Nat Immunol 18(2):123–131
Ghersi-Egea JF, Strazielle N, Catala M, Silva-Vargas V, Doetsch F, Engelhardt B (2018) Molecular anatomy and functions of the choroidal blood-cerebrospinal fluid barrier in health and disease. Acta Neuropathol 135(3):337–361
Van Hove H, Martens L, Scheyltjens I et al (2019) A single-cell atlas of mouse brain macrophages reveals unique transcriptional identities shaped by ontogeny and tissue environment. Nat Neurosci 22(6):1021–1035
Li Q, Barres BA (2018) Microglia and macrophages in brain homeostasis and disease. Nat Rev Immunol 18(4):225–242
Konishi H, Kobayashi M, Kunisawa T et al (2017) Siglec-H is a microglia-specific marker that discriminates microglia from CNS-associated macrophages and CNS-infiltrating monocytes. Glia 65(12):1927–1943
Ivan DC, Walthert S, Berve K, Steudler J, Locatelli G (2020) Dwellers and trespassers: mononuclear phagocytes at the borders of the central nervous system. Front Immunol 11:609921
Kierdorf K, Masuda T, Jordao MJC, Prinz M (2019) Macrophages at CNS interfaces: ontogeny and function in health and disease. Nat Rev Neurosci 20(9):547–562
Goldmann T, Wieghofer P, Jordao MJ et al (2016) Origin, fate and dynamics of macrophages at central nervous system interfaces. Nat Immunol 17(7):797–805
Rodriguez-Lorenzo S, Konings J, van der Pol S et al (2020) Inflammation of the choroid plexus in progressive multiple sclerosis: accumulation of granulocytes and T cells. Acta Neuropathol Commun 8(1):9
Serot JM, Foliguet B, Bene MC, Faure GC (1997) Ultrastructural and immunohistological evidence for dendritic-like cells within human choroid plexus epithelium. NeuroReport 8(8):1995–1998
Kaur C, Rathnasamy G, Ling EA (2016) The choroid plexus in healthy and diseased brain. J Neuropathol Exp Neurol 75(3):198–213
Praetorius J, Damkier HH (2017) Transport across the choroid plexus epithelium. Am J Physiol Cell Physiol 312(6):C673–C686
Marchetti L, Engelhardt B (2020) Immune cell trafficking across the blood-brain barrier in the absence and presence of neuroinflammation. Vasc Biol 2(1):H1–H18
Strazielle N, Creidy R, Malcus C, Boucraut J, Ghersi-Egea JF (2016) T-lymphocytes traffic into the brain across the blood-CSF barrier: evidence using a reconstituted choroid plexus epithelium. PLoS One 11(3):e0150945
Schwerk C, Tenenbaum T, Kim KS, Schroten H (2015) The choroid plexus-a multi-role player during infectious diseases of the CNS. Front Cell Neurosci 9:80
Cui J, Shipley FB, Shannon ML et al (2020) Inflammation of the embryonic choroid plexus barrier following maternal immune activation. Dev Cell 55(5):617–28 e6
Engelhardt B (2020) Maternal infection impairs fetal brain development via choroid plexus inflammation. Dev Cell 55(5):519–521
Thompson D, Sorenson J, Greenmyer J, Brissette CA, Watt JA (2020) The Lyme disease bacterium, Borrelia burgdorferi, stimulates an inflammatory response in human choroid plexus epithelial cells. PLoS One 15(7):e0234993
Ge R, Tornero D, Hirota M et al (2017) Choroid plexus-cerebrospinal fluid route for monocyte-derived macrophages after stroke. J Neuroinflammation 14(1):153
Rayasam A, Faustino J, Lecuyer M, Vexler ZS (2020) Neonatal stroke and TLR1/2 ligand recruit myeloid cells through the choroid plexus in a CX3CR1-CCR2- and context-specific manner. J Neurosci 40(19):3849–3861
Demeestere D, Libert C, Vandenbroucke RE (2015) Therapeutic implications of the choroid plexus-cerebrospinal fluid interface in neuropsychiatric disorders. Brain Behav Immun 50:1–13
Shimada A, Hasegawa-Ishii S (2021) Increased cytokine expression in the choroid plexus stroma and epithelium in response to endotoxin-induced systemic inflammation in mice. Toxicol Rep 8:520–528
Balusu S, Van Wonterghem E, De Rycke R et al (2016) Identification of a novel mechanism of blood-brain communication during peripheral inflammation via choroid plexus-derived extracellular vesicles. EMBO Mol Med 8(10):1162–1183
Marques F, Sousa JC (2015) The choroid plexus is modulated by various peripheral stimuli: implications to diseases of the central nervous system. Front Cell Neurosci 9:136
Simpson S, Preston D, Schwerk C, Schroten H, Blazer-Yost B (2019) Cytokine and inflammatory mediator effects on TRPV4 function in choroid plexus epithelial cells. Am J Physiol Cell Physiol 317(5):C881–C893
Medzhitov R (2007) TLR-mediated innate immune recognition. Semin Immunol 19(1):1–2
Coorens M, Schneider VAF, de Groot AM et al (20) Cathelicidins inhibit Escherichia coli-induced TLR2 and TLR4 activation in a viability-dependent manner. J Immunol (Baltimore, Md : 1950) 199(4):1418–1428
Marques F, Sousa JC, Coppola G et al (2009) Kinetic profile of the transcriptome changes induced in the choroid plexus by peripheral inflammation. J Cereb Blood Flow Metab 29(5):921–932
Mottahedin A, Joakim Ek C, Truve K, Hagberg H, Mallard C (2019) Choroid plexus transcriptome and ultrastructure analysis reveals a TLR2-specific chemotaxis signature and cytoskeleton remodeling in leukocyte trafficking. Brain Behav Immun 79:216–227
Miyake K (2007) Innate immune sensing of pathogens and danger signals by cell surface Toll-like receptors. Semin Immunol 19(1):3–10
Ehrchen JM, Sunderkotter C, Foell D, Vogl T, Roth J (2009) The endogenous Toll-like receptor 4 agonist S100A8/S100A9 (calprotectin) as innate amplifier of infection, autoimmunity, and cancer. J Leukoc Biol 86(3):557–566
Yang B, Zhou Z, Li X, Niu J (2016) The effect of lysophosphatidic acid on Toll-like receptor 4 expression and the nuclear factor-κB signaling pathway in THP-1 cells. Mol Cell Biochem 422(1–2):41–49
Kwon MS, Woo SK, Kurland DB et al (2015) Methemoglobin is an endogenous toll-like receptor 4 ligand-relevance to subarachnoid hemorrhage. Int J Mol Sci 16(3):5028–5046
Fang H, Wu Y, Huang X et al (2011) Toll-like receptor 4 (TLR4) is essential for Hsp70-like protein 1 (HSP70L1) to activate dendritic cells and induce Th1 response. J Biol Chem 286(35):30393–30400
Tsan MF, Gao B (2004) Endogenous ligands of Toll-like receptors. J Leukoc Biol 76(3):514–519
Gao C, Du H, Hua Y, Keep RF, Strahle J, Xi G (2014) Role of red blood cell lysis and iron in hydrocephalus after intraventricular hemorrhage. J Cereb Blood Flow Metab 34(6):1070–1075
Fejes Z, Erdei J, Pocsi M et al (2020) Elevated pro-inflammatory cell-free microRNA levels in cerebrospinal fluid of premature infants after intraventricular hemorrhage. Int J Mol Sci 21(18)
Berkes J, Viswanathan VK, Savkovic SD, Hecht G (2003) Intestinal epithelial responses to enteric pathogens: effects on the tight junction barrier, ion transport, and inflammation. Gut 52(3):439–451
Wilson R, Alton E, Rutman A et al (1987) Upper respiratory tract viral infection and mucociliary clearance. Eur J Respir Dis 70(5):272–279
Doyle WJ, Skoner DP, Hayden F, Buchman CA, Seroky JT, Fireman P (1994) Nasal and otologic effects of experimental influenza A virus infection. Ann Otol Rhinol Laryngol 103(1):59–69
Karimy JK, Kahle KT, Kurland DB, Yu E, Gerzanich V, Simard JM (2015) A novel method to study cerebrospinal fluid dynamics in rats. J Neurosci Methods 241:78–84
Liu G, Mestre H, Sweeney AM et al (2020) Direct Measurement of Cerebrospinal Fluid Production in Mice. Cell Rep 33(12):108524
Chen Z, Jalabi W, Shpargel KB et al (2012) Lipopolysaccharide-induced microglial activation and neuroprotection against experimental brain injury is independent of hematogenous TLR4. J Neurosci 32(34):11706–11715
Demeestere D, Libert C, Vandenbroucke RE (2015) Clinical implications of leukocyte infiltration at the choroid plexus in (neuro)inflammatory disorders. Drug Discov Today 20(8):928–941
Kleine TO, Benes L (2006) Immune surveillance of the human central nervous system (CNS): different migration pathways of immune cells through the blood-brain barrier and blood-cerebrospinal fluid barrier in healthy persons. Cytometry A 69(3):147–151
Cox KH, Cox ME, Woo-Rasberry V, Hasty DL (2012) Pathways involved in the synergistic activation of macrophages by lipoteichoic acid and hemoglobin. PLoS One 7(10):e47333
Wang YC, Zhou Y, Fang H et al (2014) Toll-like receptor 2/4 heterodimer mediates inflammatory injury in intracerebral hemorrhage. Ann Neurol 75(6):876–889
Alessi DR, Zhang J, Khanna A, Hochdorfer T, Shang Y, Kahle KT (2014) The WNK-SPAK/OSR1 pathway: master regulator of cation-chloride cotransporters. Sci Signal 7(334):re3
Thastrup JO, Rafiqi FH, Vitari AC et al (2012) SPAK/OSR1 regulate NKCC1 and WNK activity: analysis of WNK isoform interactions and activation by T-loop trans-autophosphorylation. Biochem J 441(1):325–337
Yan Y, Nguyen H, Dalmasso G, Sitaraman SV, Merlin D (2007) Cloning and characterization of a new intestinal inflammation-associated colonic epithelial Ste20-related protein kinase isoform. Biochim Biophys Acta 1769(2):106–116
Yan Y, Merlin D (2008) Ste20-related proline/alanine-rich kinase: a novel regulator of intestinal inflammation. World J Gastroenterol 14(40):6115–6121
Yan Y, Dalmasso G, Nguyen HT, Obertone TS, Sitaraman SV, Merlin D (2009) Ste20-related proline/alanine-rich kinase (SPAK) regulated transcriptionally by hyperosmolarity is involved in intestinal barrier function. PLoS One 4(4):e5049
Yan Y, Laroui H, Ingersoll SA et al (2011) Overexpression of Ste20-related proline/alanine-rich kinase exacerbates experimental colitis in mice. J Immunol 187(3):1496–1505
Zhang Y, Viennois E, Xiao B et al (2013) Knockout of Ste20-like proline/alanine-rich kinase (SPAK) attenuates intestinal inflammation in mice. Am J Pathol 182(5):1617–1628
Lin TJ, Yang SS, Hua KF, Tsai YL, Lin SH, Ka SM (2016) SPAK plays a pathogenic role in IgA nephropathy through the activation of NF-kappaB/MAPKs signaling pathway. Free Radic Biol Med 99:214–224
Polek TC, Talpaz M, Spivak-Kroizman T (2006) The TNF receptor, RELT, binds SPAK and uses it to mediate p38 and JNK activation. Biochem Biophys Res Commun 343(1):125–134
Wu CP, Huang KL, Peng CK, Lan CC (2020) Acute Hyperglycemia Aggravates Lung Injury via Activation of the SGK1-NKCC1 Pathway. Int J Mol Sci 21(13)
Hung CM, Peng CK, Yang SS, Shui HA, Huang KL (2020) WNK4-SPAK modulates lipopolysaccharide-induced macrophage activation. Biochem Pharmacol 171:113738
Piechotta K, Lu J, Delpire E (2002) Cation chloride cotransporters interact with the stress-related kinases Ste20-related proline-alanine-rich kinase (SPAK) and oxidative stress response 1 (OSR1). J Biol Chem 277(52):50812–50819
Xu H, Fame RM, Sadegh C et al (2021) Choroid plexus NKCC1 mediates cerebrospinal fluid clearance during mouse early postnatal development. Nat Commun 12(1):447
Walcott BP, Iorgulescu JB, Stapleton CJ, Kamel H (2015) Incidence, Timing, and Predictors of Delayed Shunting for Hydrocephalus After Aneurysmal Subarachnoid Hemorrhage. Neurocrit Care 23(1):54–58
Sharma D, Shah I, Patel S (2016) Late onset hydrocephalus in children with tuberculous meningitis. J Family Med Prim Care 5(4):873–874
Schiefenhövel F, Immig K, Prodinger C, Bechmann I (2017) Indications for cellular migration from the central nervous system to its draining lymph nodes in CD11c-GFP+ bone-marrow chimeras following EAE. Exp Brain Res 235(7):2151–2166
Louveau A, Plog BA, Antila S, Alitalo K, Nedergaard M, Kipnis J (2017) Understanding the functions and relationships of the glymphatic system and meningeal lymphatics. J Clin Invest 127(9):3210–3219
Louveau A, Smirnov I, Keyes TJ et al (2015) Structural and functional features of central nervous system lymphatic vessels. Nature 523(7560):337–341
Eming SA, Hammerschmidt M, Krieg T, Roers A (2009) Interrelation of immunity and tissue repair or regeneration. Semin Cell Dev Biol 20(5):517–527
Liu Q, Zhou YH, Yang ZQ (2016) The cytokine storm of severe influenza and development of immunomodulatory therapy. Cell Mol Immunol 13(1):3–10
Lawrence SM, Corriden R, Nizet V (2020) How Neutrophils Meet Their End. Trends Immunol 41(6):531–544
McAllister JP, Guerra MM, Ruiz LC et al (2017) Ventricular zone disruption in human neonates with intraventricular hemorrhage. J Neuropathol Exp Neurol 76(5):358–375
Lewin JJ 3rd, Cook AM, Gonzales C et al (2019) Current practices of intraventricular antibiotic therapy in the treatment of meningitis and ventriculitis: results from a multicenter retrospective cohort study. Neurocrit Care 30(3):609–616
Rice TW, Wheeler AP, Bernard GR et al (2010) A randomized, double-blind, placebo-controlled trial of TAK-242 for the treatment of severe sepsis. Crit Care Med 38(8):1685–1694
Liu SF, Ye X, Malik AB (1999) Inhibition of NF- B activation by pyrrolidine dithiocarbamate prevents in vivo expression of proinflammatory genes. Circulation 100(12):1330–1337
Hu Y, Wang Z, Pan S et al (2017) Melatonin protects against blood-brain barrier damage by inhibiting the TLR4/ NF-κB signaling pathway after LPS treatment in neonatal rats. Oncotarget 8(19):31638–31654
Robinson S, Conteh FS, Oppong AY et al (2018) Extended combined neonatal treatment with erythropoietin plus melatonin prevents posthemorrhagic hydrocephalus of prematurity in rats. Front Cell Neurosci 12:322
Gu C, Hao X, Li J, Hua Y, Keep RF, Xi G (2019) Effects of minocycline on epiplexus macrophage activation, choroid plexus injury and hydrocephalus development in spontaneous hypertensive rats. J Cereb Blood Flow Metab 39(10):1936–1948
Erker T, Brandt C, Tollner K et al (2016) The bumetanide prodrug BUM5, but not bumetanide, potentiates the antiseizure effect of phenobarbital in adult epileptic mice. Epilepsia
Pressler RM, Boylan GB, Marlow N et al (2015) Bumetanide for the treatment of seizures in newborn babies with hypoxic ischaemic encephalopathy (NEMO): an open-label, dose finding, and feasibility phase 1/2 trial. Lancet Neurol 14(5):469–477
Lemonnier E, Degrez C, Phelep M et al (2012) A randomised controlled trial of bumetanide in the treatment of autism in children. Transl Psychiatry 2:e202
Lemonnier E, Ben-Ari Y (2010) The diuretic bumetanide decreases autistic behaviour in five infants treated during 3 months with no side effects. Acta Paediatr 99(12):1885–1888
Lemonnier E, Villeneuve N, Sonie S et al (2017) Effects of bumetanide on neurobehavioral function in children and adolescents with autism spectrum disorders. Transl Psychiatry 7(3):e1056
Zhang J, Bhuiyan MIH, Zhang T et al (2020) Modulation of brain cation-Cl(-) cotransport via the SPAK kinase inhibitor ZT-1a. Nat Commun 11(1):78
Funding
KTK is supported by the NIH (RO1NS109358-04) and the Hydrocephalus Association.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Robert, S.M., Reeves, B.C., Marlier, A. et al. Inflammatory hydrocephalus. Childs Nerv Syst 37, 3341–3353 (2021). https://doi.org/10.1007/s00381-021-05255-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-021-05255-z