Abstract
Objective
In neonatal lambs, the quantitative evidence suggests that a significant volume of cranial CSF drainage is associated with transport along olfactory nerves with absorption primarily into extracranial lymphatics in the paranasal region. Arachnoid granulations appear to be poorly developed at this level of development and their function is unknown. In this report, we tested whether a CSF protein tracer (131I-human serum albumin) could transport directly into the superior sagittal sinus of newborn lambs.
Methods and results
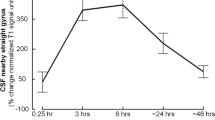
The concentration of the tracer administered into the CSF compartment was measured in the confluence of the intracranial venous sinuses (torcula) and in the peripheral blood (inferior vena cava). Enrichment of the CSF tracer in the cranial venous system was most evident when the CSF-venous sinus pressure gradients approached 20–30 cm H2O.
Conclusion
The data suggests that neonatal CSF can be absorbed directly into the cranial venous system. However, contrary to the classical view, this route may represent an auxiliary system that is recruited to compliment lymphatic transport when intracranial pressures are very high.





Similar content being viewed by others
References
Andres KH, von During M, Muszynski K, Schmidt RF (1987) Nerve fibres and their terminals of the dura mater encephali of the rat. Anat Embryol 175:289–301
Boulton M, Young A, Hay JB, Armstrong D, Flessner M, Schwartz M, Johnston M (1996) Drainage of CSF through lymphatic pathways and arachnoid villi in sheep: measurement of 125I-albumin clearance. Neuropathol Appl Neurobiol 22:325–333
Boulton M, Flessner M, Armstrong D, Hay JB, Johnston M (1997) Lymphatic drainage of the CNS: effects of lymphatic diversion/ligation on CSF protein transport to plasma. Am J Physiol 272:R1613–R1619
Boulton M, Flessner M, Armstrong D, Hay J, Johnston M (1998) Determination of volumetric cerebrospinal fluid absorption into extracranial lymphatics in sheep. Am J Physiol 274:R88–R96
Bozanovic-Sosic R, Mollanji R, Johnston MG (2001) Spinal and cranial contributions to total cerebrospinal fluid transport. Am J Physiol 281:R909–R916
Bradbury MWB, Cserr HF (1985) In: Johnston MG (ed) Drainage of cerebral interstitial fluid and of cerebrospinal fluid into lymphatics. (Experimental biology of the lymphatic circulation 9). Elsevier, Amsterdam, pp 355–394
Caversaccio M, Peschel O, Arnold W (1996) The drainage of cerebrospinal fluid into the lymphatic system of the neck in humans. Otorhinolaryngol Relat Spec 58:164–166
Chen G, Castro WL, Chow H, Reichlin S (1997) Clearance of 125I-labeled interleukin-6 from brain into blood following intracerebroventricular injection in rats. Endocrinology 138:4830–4836
Clark WL (1920) On the Pacchionian bodies. J Anat 55:40–48
Csanda E, Obal F, Obal F Jr (1983) In: Foldi M, Casley-Smith JR (eds) Central nervous system and lymphatic system. (Lymphangiology.) Schattauer, Stuttgart, pp 475–508
Egnor M, Zheng L, Rosiello A, Gutman F, Davis R (2002) A model of pulsations in communicating hydrocephalus. Pediatr Neurosurg 36:281–303
Fox RJ, Walji AH, Mielke B, Petruk KC, Aronyk KE (1996) Anatomic details of intradural channels in the parasagittal dura: a possible pathway for flow of cerebrospinal fluid. Neurosurgery 39:84–91
Gomez DG, Potts DG, Deonarine V (1974) Arachnoid granulations of the sheep. Arch Neurol 30:169–175
Gomez DG, Ehrmann JE, Potts DG, Pavese AM, Gilanian A (1983) The arachnoid granulation of the newborn human: an ultrastructural study. Int J Dev Neuroscience 1:139–147
Johnston MG, Papaiconomou C (2002) Cerebrospinal fluid transport: a lymphatic perspective. News Physiol Sci 17:227–230
Kida S, Pantazis A, Weller RO (1993) CSF drains directly from the subarachnoid space into nasal lymphatics in the rat. Anatomy, histology and immunological significance. Neuropathol Appl Neurobiol 19:480–488
Löwhagen P, Johansson BB, Nordborg C (1994) The nasal route of cerebrospinal fluid drainage in man. A light-microscopic study. Neuropathol Appl Neurobiol 20:543–550
Mann JD, Butler AB, Johnson RN, Bass HB (1979) Clearance of macromolecular and particulate substances from the cerebrospinal fluid system of the rat. J Neurosurg 50:343–348
McComb JG, Davson H, Hyman S, Weiss MH (1982) Cerebrospinal fluid drainage as influenced by ventricular pressure in the rabbit. J Neurosurg 56:790–797
McComb JG, Hyman S, Weiss MH (1984) In: Shapiro K, Marmarou A, Portnoy H (eds) Lymphatic drainage of cerebrospinal fluid in the cat. (Hydrocephalus.) Raven, New York, pp 83–98
Mollanji R, Bozanovic-Sosic R, Silver I, Kim C, Li B, Midha R, Johnston MG (2001) Intracranial pressure accommodation is impaired by blocking pathways leading to extracranial lymphatics. Am J Physiol 280:R1573–R1581
Mollanji R, Papaiconomou C, Boulton M, Midha R, Johnston MG (2001) Comparison of cerebrospinal fluid transport in fetal and adult sheep. Am J Physiol 281:R1215–R1223
Mollanji R, Bozanovic-Sosic R, Zakharov A, Makarian L, Johnston MG (2002) Blocking cerebrospinal fluid absorption through the cribriform plate increases resting intracranial pressure. Am J Physiol 282:R1593–R1599
Osaka K, Handa H, Matsumoto S, Yasuda M (1980) Development of the cerebrospinal fluid pathway in the normal and abnormal human embryos. Childs Brain 6:26–38
Papaiconomou C, Zakharov A, Bozanovic-Sosic R, Johnston M (2002) Does neonatal cerebrospinal fluid absorption occur via arachnoid projections or extracranial lymphatics? Am J Physiol 283:R869–R876
Peńa A, Harris NG, Bolton MD, Czosnyka M, Pickard JD (2002) Communicating hydrocephalus: the biomechanics of progressive ventricular enlargement revisited. Acta Neurochir 81:59–63
Pollay M, Welch K (1962) The function and structure of canine arachnoid villi. J Surg Res II:307–311
Potts DG, Reilly KF, Deonarine V (1972) Morphology of the arachnoid villi and granulations. Radiology 105:333–341
Silver I, Li B, Szalai JP, Johnston M (1999) Relationship between intracranial pressure and cervical lymphatic pressure and flow in sheep. Am J Physiol 277:R1712–R1717
Silver I, Kim C, Mollanji R, Johnston M (2002) Cerebrospinal fluid outflow resistance in sheep: impact of blocking cerebrospinal fluid transport through the cribriform plate. Neuropathol Appl Neurobiol 28:67–74
Welch K, Friedman V (1960) The cerebrospinal fluid valves. Brain 83:454–469
Welch K, Pollay M (1961) Perfusion of particles through arachnoid villi of the monkey. Am J Physiol 201:651–654
Weller RO, Kida S, Zhang ET (1992) Pathways of fluid drainage from the brain—morphological aspects and immunological significance in rat and man. Brain Pathol 2:277–284
Acknowledgements
The authors wish to thank Ms. Dianna Armstrong and Ms. Beverley Young for technical assistance. This research was funded by a grant from the Canadian Institutes of Health Research (CIHR) and a CIHR/Spina Bifida and Hydrocephalus Association of Canada Doctoral Research Award to C.P.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Papaiconomou, C., Zakharov, A., Azizi, N. et al. Reassessment of the pathways responsible for cerebrospinal fluid absorption in the neonate. Childs Nerv Syst 20, 29–36 (2004). https://doi.org/10.1007/s00381-003-0840-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00381-003-0840-z