Abstract
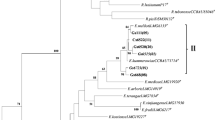
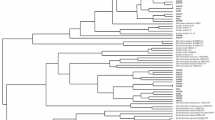
The aim of this work was to investigate the genetic diversity, symbiotic effectiveness, drought tolerance, and indole acetic acid production of indigenous rhizobial populations in the Parque Chaqueño of Argentina able to nodulate Prosopis alba, the dominant forest tree of this region. The populations were sampled at five locations from the Arid, Semi-arid, and Humid Chaco in the Parque Chaqueño region. A set of rhizobial strains able to nodulate P. alba was obtained and selected based on their molecular diversity. Data obtained by BOX-PCR indicated that the highest molecular variability was observed in rhizobial isolates from Semi-arid Chaco. High level of indolic compound production and tolerance to osmotic treatment were significantly (p ≤ 0.05) correlated with water restrictions of the environments where the strains belonged. A small set of rhizobial strains that stimulate P. alba growth was selected from a large group of strains. The strains were identified by 16S rDNA sequencing as belonging to the genera Mesorhizobium, Bradyrhizobium, and Ensifer. To our knowledge, this is the first report of P. alba nodulation by strains other than Mesorhizobium chacoense, which was already described for the Parque Chaqueño.







Similar content being viewed by others
References
Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K (1987) Current protocols in molecular biology. Greene Publishing Associates/Wiley Interscience, New York
Baca BE, Elmerich C (2007) Microbial production of plants hormones by microorganisms. In: Elmerich C, Newton W (eds) Associative nitrogen-fixation Bacteria and Cyanobacteria. IV Series Nitrogen Fixation: Origins, Applications, and Research Progress. Springer, Dordrecht, pp 113–137
Balzarini MG, Di Rienzo JA (2011) InfoGen version 2011. FCA, Universidad Nacional de Córdoba, Argentina. URL http://www.info-gen.com.ar
Balzarini MG, Bruno C, Fernandez E (2011) Multivariate analysis in phytopathology: options and opportunities in data mining to face new molecular information. In: Rodriguez Herrera R, Aguilar C, Simpson-Williamson J, Gutierrez Sanchez G (eds) Phytopathology in the omics era. Research Signpost, Kerala, pp 1–20
Broughton WJ, Dilworth MJ (1971) Control of leghemoglobin synthesis in snake beans. Biochem J 125:1075–1080
Burkart A (1976) A monograph of the genus Prosopis (Leguminosae subfam. Mimosoideae): catalogue of the recognized species of Prosopis. J Arnold Arbor 57:450–525
Cesco S, Mimmo T, Tonon G, Tomasi N, Pinton R, Terzano R, Neumann G, Weisskopf L, Renella G, Landi L, Nannipieri P (2012) Plant-borne flavonoids released into the rhizosphere: impact on soil bio-activities related to plant nutrition. A review. Biol Fertil Soils 48:123–149
Chang WS, Franck WL, Cytryn E, Jeong S, Joshi T, Emerich DW, Sadowsky MJ, Xu D, Stacey G (2007) An oligonucleotide microarray resource for transcriptional profiling of Bradyrhizobium japonicum. MPMI 20:1298–1307
Di Rienzo JA, Casanoves F, Balzarini MG, Gonzalez L, Tablada M, Robledo CW (2012) InfoStat versión 2012. Grupo InfoStat, FCA, Universidad Nacional de Córdoba, Argentina. URL http://www.infostat.com.ar
Gel Compar Applied Maths NV
Glickman E, Dessaux Y (1995) A critical examination of the specificity of the Salkowski reagent for indolic compounds produced by phytopathogenic bacteria. Appl Environ Microbiol 61:793–796
Haukka K, Lindstro K, Young P (1998) Three phylogenetic groups of nodA and nifH genes in Sinorhizobium and Mesorhizobium isolates from leguminous trees growing in Africa and Latin America. Appl Environ Microbiol 64:419–426
Iglesias O, Rivas R, Garcia-Fraile P, Abril A, Mateos P, Martinez-Molina E, Velázquez E (2007) Genetic characterization of fast-growing rhizobia able to nodulate Prosopis alba in North Spain. FEMS Microbiol Lett 277:210–216
Laranjo M, Oliveira S (2011) Tolerance of Mesorhizobium type strains to different environmental stresses. Antonie van Leeuwenhoek 99:651–662
Melchiorre M, de Luca M, Gonzalez Anta G, Suarez P, Lopez C, Lascano R, Racca R (2011) Bradyrhizobia strains isolation from field-grown soybean plants in Argentina and evaluation of their performance as improved inoculants. Biol Fertil Soils 47:81–89
Mottura M (2006) Development of microsatellites in Prosopis spp and their application to study the reproduction system. PhD thesis. Georg-August University of Göttingen, Germany
Räsänen LR, Sprent JI, Lindstrom K (2001) Symbiotic properties of Sinorhizobia isolated from Acacia and Prosopis nodules in Sudan and Senegal. Plant Soil 235:193–210
Shannon CE, Weaver W (1949) The mathematical theory of communication. University of Illinois Press, Urbana, pp 3–24
Spaepen S, Vanderleyden J, Remans R (2007) Indole-3-acetic acid in microbial and microorganism–plant signaling. FEMS Microbiol Rev 31:425–448
Sprent J, James EK (2007) Legume evolution: where do nodules and mycorrhizas fit in? Plant Physiol 144:575–581
Steenhoudt O, Vanderleyden J (2000) Azospirillum, a free-living nitrogen-fixing bacterium closely associated with grasses: genetic, biochemical and ecological aspects. FEMS Microbiol Rev 24:487–506
Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S (2011) MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance and maximum parsimony methods. Mol Biol Evol 28:2731–2739
van der Weele CM, Spollen WG, Sharp RE, Baskin TI (2000) Growth of Arabidopsis thaliana seedlings under water deficit studied by control of water potential in nutrient–agar media. J Exp Bot 51:1555–1562
Velázquez E, Igual JM, Willems A, Fernandez MP, Munoz E, Mateos PF, Abril A, Toro N, Normand P, Cervantes E, Gillis M, Martinez-Molina E (2001) Mesorhizobium chacoense sp. nov., a novel species that nodulates Prosopis alba in the Chaco Arido region (Argentina). Int J Syst Evol Microbiol 51:1011–1021
Versalovic J, Schneider M, de Bruijn F, Lupski JR (1994) Genomic fingerprinting of bacteria using repetitive sequence-based polymerase chain reaction. Methods Mol Cell Biol 5:25–40
Verzino G, Carranza C, Ledesma M, Joseau J, Di Rienzo J (2003) Adaptive genetic variation of Prosopis chilensis (Mol) Stuntz. Preliminary results from one test-site. For Ecol Manage 175:119–129
Vincent JM (1970) The cultivation, isolation and maintenance of rhizobia. In: Vincent JM (ed) A manual for the practical study of root-nodule bacteria. Blackwell Scientific, Oxford, pp 1–13
Zahran H (2001) Rhizobia from wild legumes: diversity, taxonomy, ecology, nitrogen fixation and biotechnology. J Biotechnol 91:143–153
Acknowledgments
The authors thank Dr. Cecilia Bruno (Universidad Nacional de Córdoba) for her assistance in statistical data analysis, Diego Lopez Lauenstein for soil sampling, Franco Fernandez for his assistance in sequence analysis, and Paola Suarez and Daniela Gomez (IFRGV-INTA) for technical assistance. Anibal Verga, Nacira Muñoz, and Ramiro Lascano (IFRGV-INTA) critically reviewed the manuscript and helped in the discussion of data. We also thank translator Jorgelina Brasca for correcting the English style. L.C.H.D. is FCEFN–UNC student, P.G. and E.R. are INTA researchers, and M.M. is INTA and CONICET researcher. This work was supported by INTA-PNFOR-044341 and Secyt-UNC 162/12 projects. The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 23 kb)
Rights and permissions
About this article
Cite this article
Chávez Díaz, L., González, P., Rubio, E. et al. Diversity and stress tolerance in rhizobia from Parque Chaqueño region of Argentina nodulating Prosopis alba . Biol Fertil Soils 49, 1153–1165 (2013). https://doi.org/10.1007/s00374-013-0814-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-013-0814-6