Abstract
Literature analysis and chemical considerations of biological phosphate solubilization have shown that the commonly used selection factor for this trait, tricalcium phosphate (TCP), is relatively weak and unreliable as a universal selection factor for isolating and testing phosphate-solubilizing bacteria (PSB) for enhancing plant growth. Most publications describing isolation of PSB employed TCP. The use of TCP usually yields many (up to several thousands per study) isolates “supposedly” PSB. When these isolates are further tested for direct contribution of phosphorus to the plants, only a very few are true PSB. Other compounds are also tested, but on a very small scale. These phosphates (P), mainly Fe-P, Al-P, and several Ca-P, are even less soluble than TCP in water. Because soils greatly vary by pH and several chemical considerations, it appears that there is no metal-P compound that can serve as the universal selection factor for PSB. A practical approach is to use a combination of two or three metal-P compounds together or in tandem, according to the end use of these bacteria—Ca-P compounds (including rock phosphates) for alkaline soils, Fe-P and Al-P compounds for acidic soils, and phytates for soils rich in organic P. Isolates with abundant production of acids will be isolated. This approach will reduce the number of potential PSB from numerous isolates to just a few. Once a potential isolate is identified, it must be further tested for direct contribution to P plant nutrition and not necessarily to general growth promotion, as commonly done because promotion of growth, even by PSB, can be the outcome of other mechanisms. Isolates that do not comply with this general sequence of testing should not be declared as PSB.
Similar content being viewed by others
Introduction and background
Apart from the basic need for phosphorus in plant nutrition, there are three reasons why phosphate fertilization is a major agricultural research topic: (1) the price of fertilizers skyrocketed in recent times, making P fertilizers beyond the reach of many farmers in developing countries, (2) competition for high quality rock phosphate from other industries, such as food preservatives, anticorrosion agents, cosmetics, fungicides, ceramics, water treatment, and metallurgy, all providing costlier products, and (3) sources of high quality phosphates are rapidly depleted and expected to be exhausted in less than 100 years (Middleton 2003). Annual consumption of phosphate rock has approached 150 million metric tons; about 95 % of this production is used in the fertilizer industry (Dorozhkin 2011).
Inorganic P occurs in soil, mostly in insoluble mineral complexes, some of them appearing after frequent application of chemical fertilizers. These insoluble, precipitated forms cannot be absorbed by plants (Rengel and Marschner 2005). Organic matter is also an important reservoir of immobilized P that accounts for 20–80 % of P in soils (Richardson 1994). Only 0.1 % of the total P exists in a soluble form available for plant uptake (Zou et al. 1992).
The original source of most soil and plant P is apatite (Ahn 1993). Under natural conditions in soil and in seawater, P rapidly precipitates as forms of sparingly soluble complexes of different kinds of phosphates. The most common ones in acid agricultural soils are variscite (AlPO4·2H2O), followed by strengite (FePO4·2H2O). Both are very stable minerals (Richardson 2001). In alkaline soils having abundant calcium, there is no detectable orthophosphate (PO 3−4 , Pi) (Goldstein et al. 1999). The most stable minerals are calcium phosphates, which are, in order of decreasing solubilities, dicalcium phosphate dihydrate (brushite) CaHPO4·2H2O > anhydrous dicalcium phosphate (monetite) CaHPO4 > octacalcium phosphate Ca8H2(PO4)6·5H2O > tricalcium phosphate Ca3(PO4)2 > hydroxyapatite Ca5(PO4)3OH > fluorapatite Ca5(PO4)3F (Haynes 1982; Wang and Nancollas 2008; Dorozhkin 2011). Stable forms for organic P are phytate (dodecasodium inositol hexaphosphate, Na12C6H6P6O24) and for rock phosphate (sedimentary rock containing phosphate minerals, phosphorite) are apatite, fluorapatite, and hydroxyapatite (Hoffland 1992). The low solubility of these stable minerals makes them unavailable for plants (using soluble Pi for growth). This results in frequent shortage of Pi for plant nutrition, even though the soil may contain a high level of total P (Merbach et al. 2010).
For the above reasons, agricultural research focused on the following: (1) low-grade rock phosphate (9–11 % P2O5 or less) as a source of fertilizer in the future because low-grade ore is available worldwide in large quantities (Rajan et al. 1996; Bationo et al. 1997) and (2) other sources of phosphate, such as struvite derived from wastewater treatment (de-Bashan and Bashan 2004). This trend especially happens in developing countries where P availability for crops is more acute. In rock phosphate used as fertilizer, where its insoluble phosphate is almost unavailable for plant growth, phosphate-solubilizing microorganisms are used to transform the insoluble phosphate into available soluble phosphate. This is the most common and logical approach (Reyes et al. 2001; Whitelaw 1999; Richardson 2001; Rodriguez et al. 2006).
Phosphate-solubilizing bacteria
The history and first experimental findings pointing to the important role of soil microorganisms in solubilizing phosphate minerals, making Pi available to plants, is at least century-old knowledge, as outlined in the landmark paper by Gerretsen (1948) and later by Goldstein (Goldstein 2007; Goldstein and Krishnaraj 2007). For example, one of the earliest reports date back to 1908 (Sackett et al. 1908; cited from Greaves 1922).
In recent years, many publications presented a very large number of new phosphate-solubilizing bacteria (PSB). Some involved isolation of thousands of new strains (for example, Mehta and Nautiyal 2001; Peix et al. 2003, 2004; Chung et al. 2005; Chen et al. 2006; Reyes et al. 2006; Gulati et al. 2008; Jorquera et al. 2008) or were offered directly by inoculant companies without any publication record. High percentage of those were preliminary studies, conducted solely in vitro, and lacking plant and field application. Many of these studies assumed that in vitro P solubilization capacity will translate into available P for plant nutrition under usual growing conditions in soil. Some optimistic reports notwithstanding (Kumar and Narula 1999; Harris et al. 2006), this is by far not the case (Rengel and Marschner 2005). The literature abounds with microbiological reports on successful in vitro solubilization of P that could not be repeated under field conditions (Gyaneshwar et al. 2002; Rengel and Marschner 2005).
To isolate a suitable potential PSB, a defined selective medium lacking available sources of soluble P, apart from insoluble P, is the right strategy. Theoretically, this will ensure that any isolate growing in this medium has phosphate-solubilizing ability. Such a growth medium was initially proposed in 1948 by Pikovskaya (1948). This medium contains, as its sole selection factor, tricalcium phosphate [TCP, Ca3(PO4)2] with a low-medium rate of solubilization (Haynes 1982). With time, TCP became the selection factor for isolating new PSB or for demonstrating phosphate-solubilizing capacity in numerous studies (Kumar and Narula 1999; Rodriguez and Fraga 1999; Katiyar and Goel 2003; Chen et al. 2006; Rajkumar et al. 2006; Son et al. 2006; Ahmad et al. 2008; Jorquera et al. 2008; Oliveira et al. 2009; Park et al. 2010; Liu et al. 2011; Table 1). Later, the medium was modified, but the modification still contains TCP as a selection factor (India’s National Botanical Research Institute (NBRIP) phosphate growth medium; Nautiyal 1999). This medium was proposed as a suitable and appropriate medium for the task of isolating and testing PSB and is widely used. In addition to TCP, other P compounds are used for isolating and testing PSB, such as rock phosphate (hydroxyapatite; Reyes et al. 2006), dicalcium phosphate (Chabot et al. 1996; Antoun et al. 1998; Peix et al. 2003, 2004), and phytate (Chabot et al. 1996; Unno et al. 2005), but used to a far lesser extent.
It appears that TCP, although a potentially insoluble P, is not hard to dissolve, compared with other harder-to-dissolve phosphates. Pérez et al. (2007) isolated 130 strains capable of solubilizing TCP from thousands of colonies developed on NBRIP medium. None of those showed solubilizing activities of iron phosphate (FePO4) or aluminum phosphate (AlPO4), which renders their usefulness largely impractical for acidic soils. From TCP plates, Park et al. (2010) isolated an efficient PSB that also solubilized FePO4 and AlPO4. Yet it solubilized TCP 10 to 50 times better. Similar results in solublizing were obtained by Song et al. (2008) and Chang and Yang (2009), using additional hydroxyapatite and rock phosphate as sources of P. When four kinds of P (TCP, AlPO4, phytate, and soybean lecithin) were compared for isolating PSB, the biggest number of strains was obtained from TCP plates and they solubilized best this P material (Oliveira et al. 2009). Comparison of several PSB Azotobacter chroococcum strains to solubilize TCP and Mussoorie (India) rock phosphate showed that the latter is hardly solubilized (Kumar and Narula 1999). Even cloned Escherichia coli, having no relation to plant nutrition, is capable of solubilizing TCP (Goldstein and Liu 1987; Kim et al. 1997, 1998). By this test, even E. coli would be considered a PSB. It appears that numerous bacterial strains that produce organic acid from metabolism of sugars, especially metabolizing glucose to strong gluconic and 2-ketogluconic acids, are capable of dissolving TCP (Mehta and Nautiyal 2001; Chen et al. 2006; Goldstein 2007; Goldstein and Krishnaraj 2007; Trivedi and Sa 2008). Therefore, all these bacteria are presumably PSB, which would be an absurd conclusion.
Finally, as mentioned in the report by Greaves (1922), TCP is several times more soluble in CO2-saturated water than in pure water, resulting in its gradual transformation to more soluble Ca salts. This happens according to the following general equation:
In summary, any TCP-based selective medium that isolated hundreds or thousands of potential PSB strains from a soil is not selective enough for the task of selection.
PSB and plant growth-promoting bacteria
With time, evidence accumulated in the literature reporting potential PSB candidates from in vitro studies that very few were also plant growth-promoting bacteria (PGPB). PSB and plant growth-promoting bacteria (PSB-PGPB) are the end-product microorganisms to be used in agriculture as inoculants and are the declared main final goal of all the PSB studies. For example, Collavino et al. (2010) found that there is no correlation between potential PSB isolated on TCP and their ability to promote plant growth. Fernández et al. (2007) tested 13 efficient TCP-solubilizing strains from soil and found that none promoted plant growth nor improved plant P nutrition. Taurian et al. (2010) tested 110 potential PSB on TCP and found only one that could promote peanut growth. El-Tarabily and Youssef (2010) isolated 129 TCP-solubilizing bacteria but only one was effective as a PSB-PGPB for mangrove seedlings. An efficient PSB-PGPB for maize exhibited much higher solubilization of TCP than for three types of rock phosphate (Gulati et al. 2010). Yu et al. (2011) isolated a large number of PSB on TCP medium yet could find only two PSB-PGPB; they propose that more insoluble P materials should be selected to isolate potential PSB-PGPB. At the same time, studies that used rock phosphate or other hard-to-dissolve phosphates were more successful in isolating PSB-PGPB, but not necessarily with phosphate solubilization as the main mechanism (de Freitas et al. 1997; Puente et al. 2004a, b, 2009a, b; Lopez et al. 2011, 2012; Baig et al. 2012). In summary, regardless of the promise of PSB, the multitude of strains isolated, and the several microbial inoculants in the marketplace, successful application in the field is still very low.
Analysis of the literature
Here, we extracted data from common scientific literature on PSB, their origin, selection factor for their isolation, and their performance as PSB-PGPB, and considered the potential chemical solubilization pathways for several hard-to-dissolve phosphates by potential PSB and those available in the rhizosphere of plants. This was done to reach an initial conclusion about which insoluble phosphate(s) is most useful to serve for isolating and testing potential PSB-PGPB.
This approach was chosen because, among the several mechanisms responsible for phosphate solubilization in soil (Illmer and Schinner 1995), production of organic acids by plant roots and their associated microbes (bacteria and fungi) plays the major role. In many bacteria, the direct oxidation pathway (also called nonphosphorylating oxidation) leads to the production of gluconic and 2-ketogluconic acids directly into the periplasmic space, where their protons are efficiently released into the extracellular medium, thus lowering its pH. These strong organic acids, having extremely low K a values, logK a = 3.4 and logK a = −2.6, respectively (Goldstein and Krishnaraj 2007), can dissolve difficult-to-dissolve calcium phosphates, such as hydroxyapatite and rock phosphate ore, such as fluorapatite. The bacteria exhibiting nonphosphorylating oxidation were designated as having the MPS+ (mineral phosphate-solubilizing) phenotype (Goldstein 1995). Goldstein (2007) proposes that conservation of the direct oxidation pathway in rhizobacteria may, at least in part, result from the mutualistic advantage provided by the MPS trait.
Acids are well known to dissolve rock phosphate (Kpomblekou and Tabatabai 1994). The most common organic acids produced by potential PSB are not only gluconic, 2-ketogluconic, citric, oxalic, succinic, propionic, and acetic but also isovaleric, heptanoic, caproic, formic, n-butyric, oxalic, and methylmalonic were detected (Chen et al. 2006; Puente et al. 2009a). In alfalfa, pea, lupin, sorghum, maize, wheat, and barley, malate and citrate are the common organic acids. These organic acids appear to be the primary components released by roots with P deficiency (Jones and Darrah 1994; Jones 1998). White lupin (Lupinus albus) exudates also contain fumaric, cis-aconitic, and trans-aconitic acids. Chickpea (garbanzo, Bengal gram; Cicer arietinum) was the only species that contained malonic acid, in addition to malic, fumaric, and cis- and trans -aconitic acids (Cawthray 2003). In general, all these acids chelate cations (mainly Ca2+, also Fe3+ and Al3+) bound to phosphate through their hydroxyl and carboxyl groups or solubilize them by the liberation of protons, thereby converting insoluble P into soluble forms that are available for plant nutrition (Kpomblekou and Tabatabai 1994).
General considerations on dissolution of phosphate minerals
To clarify the theory of rock phosphate dissolution, first, one needs to consider a simple process of dissolution of dissociating salts (including metal phosphates, the subject of this assay) in pure water or in slightly acidic or alkaline medium and disregarding any hydrolysis or complexing (see below). Solubility is defined as the maximal concentration reached under specified conditions in the binary system solid phase–solution without supersaturation. It is a thermodynamic value, which could ideally be reached for a well-crystallized stable phase in equilibrium with the solution (also see below). It is determined by the value of the solubility product for this solid phase. The solubility product is a product of the concentrations (ideally = in the ideal case activities, which can be substituted by concentrations when they are very low) of all the ions (formed upon dissociation of the salt) in the powers of their stoichiometric coefficients. As an example, for a salt M x A y (where M is a metal, A is an anion, disregarding the charges; x and y are the stoichiometric coefficients in the chemical formula), the solubility product constant (K S) for the dissociation equilibrium (2) is calculated as shown in Eq. (3).
The K S value is a constant for a stable, solid, well-crystallized phase of definite composition and can generally be used for calculating the aqueous solubility of a solid or any of the ions (M or A), if the other’s concentration in equilibrium is known. For orthophosphates, where there is also an equilibrium in solution between the protonated and nonprotonated ions (H2PO −4 , HPO 2−4 , and PO 3−4 ), which depends on the pH, the dependence of solubility on pH can also be generally calculated (Wang and Nancollas 2008; Dorozhkin 2011).
In principle, K S values may be found in the literature for various phosphate minerals for Ca phosphates (Wang and Nancollas 2008; Dorozhkin 2009, 2011) and Al and FeIII phosphates (Stumm and Morgan 1996; Jiang and Graham 1998). For AlPO4·2H2O and FePO4·2H2O (Bache 1963), different K S values (stated to be valid only at low pH) were given which are based on the dissociation scheme similar to Eq. (2), which however, additionally includes hydration water molecules and, consequently, water activity in the corresponding expression is similar to Eq. (3) for K S. This is not typical for aqueous solutions of low concentrations, where water activity can be assumed to be constant, and can be confused with calculated K S values that exclude water (Wang and Nancollas 2008). This could only complicate relevant calculations.
As mentioned above, K S, as a thermodynamic value, refers to the equilibrium conditions of the chemically uniform (homogeneous) and well-crystallized stable, solid phase only. In reality, however, there are four reasons that often make an ideal K S value virtually useless:
-
(a)
The solubility product strongly depends on the crystalline state (and on the thermodynamic stability) of a mineral (see below). Often, there is no reliable information under which conditions and for which solid phase the solubility product was measured for calculating K S.
-
(b)
In a medium where the ionic strength (i.e., background concentrations of other inert ions) is not very low (as what happens in real solutions), the activity coefficients (Wang and Nancollas 2008) may significantly differ from unity (=1) and render the values of activities and concentrations no longer equivalent (activity = concentration multiplied by activity coefficient). If the latter is not close to unity, activity becomes different from concentration (i.e., they become nonequivalent). Thus, the literature data on K S values determined at low ionic strengths become inapplicable. For example, the value of pK S (= −log10 K S) for AlPO4·2H2O at zero ionic strength (i.e., almost pure water) was reported to be pK S = 21, i.e., K S = 1.0 × 10−21 M2 (Stumm and Morgan 1996; Jiang and Graham 1998). However, at ionic strength 0.15, it was reported to be pK S = 18.3 (Duffield et al. 1991; Martin 1997), i.e., K S = 5.0 × 10−19 M2. Thus, already at such a relatively small ionic strength, which is equivalent to that of the physiological solution (0.88 % NaCl), the K S is 500 times higher than in pure water, and, consequently, the solubility of AlPO4 is increased by (500)1/2 (∼22 times).
-
(c)
Even a small impurity (that is, a small percent in the whole substance) of a less-crystalline (i.e., more amorphous) phase may give an apparent result of a higher dissolution level and/or rate (see below about the dissolution rate). Thus, the solubilization test would be wrong. It would give a higher solubilization rate than the real rate for the main substance and a higher solubility caused solely by the less-crystalline substance and more soluble impurity.
-
(d)
In real systems, when the phosphate mineral can be dissolved by an acid, the rate of dissolution/solubilization, identified as the amount of mineral dissolved per unit of time, may vary greatly, from a very high rate (very fast dissolution) to a very low rate (very slow dissolution). This solubilization rate refers not to the thermodynamics of dissolution (i.e., equilibrium state), but to the chemical kinetics. The kinetics of solubilization (dissolution) depends on a number of other parameters, one of the most important of which is the specific surface area of the mineral. This is because the solubilization reaction is a heterogeneous process (i.e., occurring between a liquid phase and a solid phase, not within a single phase) and proceeds exclusively at the surface of a mineral. In other words, its surface-exposed moieties or structural units, which are in contact with the solution, react with the ions adsorbing onto them from the solution. Therefore, the greater the surface area of a mineral sample (note that smaller crystallites and higher porosity increase the surface area per unit of mass), the faster is the rate of solubilization.
To conclude, calculations of solubilities using solubility product data (K S constants from the literature) as the data related to ideal equilibrium states in solutions would provide no information regarding the rate of true real-life solubilization. In particular, the K S values of phosphate minerals, which might be considered as a first approximation to their solubility, in view of the aforementioned, do not give any reliable information. Additionally, K S values do not take into account the complexing ability of an organic acid’s anion (see below).
Any reliable information on the ability of organic acids to dissolve poorly insoluble phosphates, including the dissolution rate, could be obtained exclusively in model experiments that should include different organic acids (pure substances) and different reference phosphate minerals, each of a definite formula (composition), chemical state (crystallinity, stability, phase homogeneity), and specific surface area. Such experimental data, obtained with the same mineral for different acids and with each acid reacting with different phosphate minerals, could be comparable enough to make valid conclusions about which acids are strong solubilizers and which mineral is more or less easy to solubilize. A database of such results could be used to determine the ability of a potential PSB to solubilize different phosphate minerals in relation to the spectrum of organic acids produced by the bacteria.
Crystallinity and solubility—general chemical considerations
While assessing and comparing the biological availability of phosphate minerals, the following important comment by Gillis et al. (1962) should be noted: “Some confusion exists in the literature because investigators not always recognized that many phosphate compounds may exist in several states of hydration and crystal modification.” This is of paramount importance with regard to biological phosphate solubilization discussed here. Different crystal modifications including not only different hydration states but also the level of crystallinity of the same substance usually have different water solubilities and different dissolution rates either in water or in acidified solutions. More amorphous phases usually have higher solubilities. This is directly related to their thermodynamic state.
As described above, the solubility (as a thermodynamic value) of a solid substance represents its concentration in solution in equilibrium with the stable solid phase. From thermodynamic considerations, the solubility tends to decrease with increasing crystallinity; therefore, most highly crystalline material has the lowest solubility. Also, more amorphous phases are commonly more rapidly dissolved. In particular, this is because of their higher specific surface area, as compared to that of a strongly crystallized phase, which favors dissolution kinetics. Therefore, for poorly soluble materials (such as phosphates), even a small admixture of an amorphous phase could dramatically affect the results of investigations on their solubility or dissolution rate and, as a consequence, misrepresent the corresponding bioavailability tests. For example, it is common that in a stable solid crystalline, especially in a natural mineral, there is a small impurity (often a few percent or even lower) of a more amorphous phase of the same substance. While dissolving, this impurity being preferentially and more rapidly dissolved may give an apparent, but not real, higher solubility. It has to be noted that such a small impurity may well be not easily detectable, being totally or almost invisible for X-ray diffraction or spectroscopic techniques. Therefore, any differences in PSB tests performed in different laboratories with the same mineral, which, in fact might differ by such impurities, could be misinterpreted as real differences between the PSB properties.
The aforementioned consideration clearly implies that, to test and compare the bioavailability of phosphate minerals for potential PSB, some stable and uniform (structurally and chemically homogeneous), well-crystallized phases should only be chosen as standards. They should have a number of defined characteristics such as spectra, thermograms, and X-ray diffraction patterns which must be checked or otherwise ensured to be valid before performing a test with PSB. This is particularly important, as for many metal phosphate minerals, different crystal modifications are known, including calcium orthophosphates (Wang and Nancollas 2008; Dorozhkin 2009, 2011). In addition, if such a test phosphate material is to be prepared in a laboratory to perform PSB tests, there should be some treatment steps developed and commonly recommended to obtain a stable and structurally homogeneous mineral phase to be used by different researchers in laboratories throughout the world.
Regarding Ca orthophosphates (including TCP because it contains PO 3−4 ), and specifically TCP, the most stable and the least soluble modification of the latter is β-Ca3(PO4)2 (with pK S = −log10 K S = 28.9 and solubility of ∼0.5 mg L−1 at 25 °C), while there is its higher temperature polymorph α-Ca3(PO4)2. Both polymorphs are stable at room temperature in the absence of humidity (Dorozhkin 2011). It has to be mentioned that neither polymorph can be precipitated from aqueous solutions (Wang and Nancollas 2008; Dorozhkin 2009, 2011). Therefore, for any PSB tests, β-TCP has to be either taken as a mineral (which might imply the presence of different impurities for minerals of different origin), which could affect the PSB tests or prepared using a high-temperature synthesis in the laboratory (which could easily be standardized).
Nevertheless, it should be noted that, among Ca-phosphate minerals, the most stable and least soluble is thought to be fluorapatite Ca10(PO4)6F2 (pK S = 120), with solubility of ∼0.2 mg L−1, stable within pH 7–12 (at 25 °C), while hydroxyapatite Ca10(PO4)6(OH)2 (pK S = 117) is close with the solubility of ∼0.3 mg L−1, stable within pH 9.5–12 (Dorozhkin 2011).
Types of microbially driven mineral phosphate dissolution processes
The processes resulting in mineral phosphate solubilization, in particular, those driven by soil microorganisms, in principle involve several chemically different reactions (Table 2):
-
1.
The most straightforward process is simple acidification of the medium as a result of proton release, for example, as the H+ antiport in the course of bacterial ammonium (NH +4 ) assimilation, by means of which the cell maintains a neutral charge, or production of inorganic acids that do not form strong complexes with Ca, Al, or FeIII and easily release protons upon dissociation (Illmer and Schinner 1995; Illmer et al. 1995; Rodriguez and Fraga 1999; Whitelaw 1999). The reaction scheme is as follows, as in the case of TCP, resulting in the formation of more soluble phosphates:
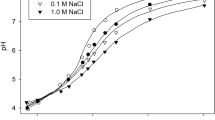
$$ {\mathrm{C}}{{\mathrm{a}}_3}{\left( {{\mathrm{P}}{{\mathrm{O}}_4}} \right)_2} + 2 {{\mathrm{H}}^{ + }} = 2{\mathrm{CaHP}}{{\mathrm{O}}_4} + {\mathrm{C}}{{\mathrm{a}}^{{2 + }}} $$(4)While the solubility of calcium phosphate increases exponentially with decreasing pH (Merbach et al. 2009), the behavior of AlPO4 and FePO4 is different (Fig. 1). The solubility of ferric phosphate decreases with lower pH down to 4.5–3.5, and aluminum phosphate has the lowest solubility within pH 5.5–4.5, for example, at pH 3.5 it is comparable with that at pH 7. Hence, acidification of the medium per se cannot account for phosphate mobilization in bacterial cultures in the cases of aluminum or ferric phosphates (Fankem et al. 2008; Merbach et al. 2009).
Fig. 1 Solubilization of phosphate from Ca, Al, and FeIII phosphates as a function of pH (adjusted using HCl or NaOH, measured after phosphate extraction). The extraction was made by mixing 100 mg of a solid phosphate in 30 mL solution under stirring for 90 min at 150 rpm. At the end of the incubation period, the solution was centrifuged at 6,000×g, its pH was measured, and the phosphate in solution was determined (information adapted from Fankem et al. (2008))
In accordance with the literature (see Fig. 1), aluminum phosphate is considerably more soluble than the corresponding ferric salt (Brosheer et al. 1954; Ishio et al. 1986; Fankem et al. 2008). Similarly, according to He et al. (2006), in 0.322 mM KH2PO4 solution, the addition of FeCl3 or AlCl3 (3.22 mM) in 100 mM acetate buffer (at pH 5.0, 22 °C) after 20 h precipitates 60 % or only 5 % of soluble inorganic phosphate, respectively.
-
2.
The formation of a metal complex in solution, where the metal ion is coordinated by an anion. Specifically, metal chelation, in the case of a chelating ligand or anion that forms two or more bonds with the metal forming a ring structure, transforms an insoluble phosphate mineral into the metal complex and releases phosphate anions. The reaction equation is as follows, as in the case of TCP, and a complexing acid H n X:
$$ {\mathrm{C}}{{\mathrm{a}}_3}{\left( {{\mathrm{P}}{{\mathrm{O}}_4}} \right)_2} + 3m{{\mathrm{H}}_n}{\mathrm{X}} = 3{\left[ {{\mathrm{Ca}}{{\mathrm{X}}_m}} \right]^{{2 - mn}}} + 2{\mathrm{HP}}{{\mathrm{O}}_4}^{{2 - }} + \left( {3mn - 2} \right){{\mathrm{H}}^{ + }}, $$(5)where m is the stoichiometric coefficient in the calcium complex formed, n is the index equal to the absolute value of the charge of the complexing anion (Xn−).
The excessive protons in Eq. (5) might be involved in parallel processes of phosphate solubilization, such as in Eq. (4), or get bound to other anions, depending on the pH of the medium. A similar process could be applicable to the less-soluble phosphates of FeIII or Al, according to the following equation:
$$ {\mathrm{FeP}}{{\mathrm{O}}_4} + m{{\mathrm{H}}_n}{\mathrm{X}} = {\left[ {{\mathrm{Fe}}{{\mathrm{X}}_m}} \right]^{{3 - mn}}} + {\mathrm{HP}}{{\mathrm{O}}_4}^{{2 - }} + \left( {mn - 1} \right){{\mathrm{H}}^{ + }}. $$(6)Note that Al and FeIII complexing (in particular, chelation) seems to be the main mechanism for the microbially driven dissolution of aluminum and ferric phosphate minerals. It is related to the well-known phenomenon of synthesis and release of a range of organic acids by many bacteria, in particular, induced by phosphate deficiency (Chen et al. 2006; Puente et al. 2009a). Phosphate may also be solubilized, not only from rock phosphate minerals but, via ligand exchange involving organic acid anions, from other minerals, such as oxides containing chemisorbed phosphate anions (Arcand and Schneider 2006).
Halo formation on solid agar, produced by developing bacterial colonies, has served as a universal indicator for phosphate solubilization by PSB for over half a century. As indicated in Table 2 (footnote a), special care should be taken, as the formation of insoluble Ca (and sometimes also Al or FeIII) complexes could solubilize phosphate but may give no solubilization halo on the agar plates. As a solution for this difficulty, such PSB test plates should be complemented by liquid culture tests or genetic characterization of potential PSB (Merbach et al. 2009). For Ca phosphate minerals, such poorly soluble complexes, preventing the formation of a solubilization halo, can be formed with oxalate, tartrate (Arcand and Schneider 2006), phytate (Evans and Pierce 1981; Grynspan and Cheryan 1983; for poorly soluble phytate complexes of Al and FeIII (He et al. 2006)), and even with citrate (Merbach et al. 2009) anions, while citrate readily dissolves AlPO4 (Martin 1997). Lobartini et al. (1998) mention that humic and, to a lesser extent, fulvic acids were useful chelating agents for Al3+ and Fe3+ and were effective in dissolving AlPO4 and FePO4. However, humic soil substances may contain organic matter–metal (Al3+ and/or Fe3+) phosphate complexes (He et al. 2006) that contribute to phosphate solubilization via ligand exchange, primarily with metal-complexing or chelating organic acid anions.
Formation of soluble and insoluble Ca, Al, or FeIII complexes with organic acid anions would dramatically reduce the concentrations of free (hydrated) ions in the medium. Thus, by lowering the solution saturation point, this would result in shifting the mineral dissolution equilibria and facilitating the dissolution processes (Welch et al. 2002).
-
3.
For the redox-active cation (in the case of FeIII phosphate only, FePO4), a preliminary metal reduction step, either directly (for Fe3+-reducing bacteria) or indirectly microbially driven (such as via release of reductive secondary metabolites), may lead to the formation of FeII. It has long been known that poorly soluble ferrous (Fe2+) salts commonly are still noticeably more soluble than the corresponding ferric (Fe3+) salts.
In particular, FeII phosphate was reported to be moderately available to plants (Gerretsen 1948). Special studies (Ghassemi and Recht 1971) showed that FeII orthophosphate (Fe3(PO4)2·8H2O, vivianite) has a sharp minimum in solubility at pH 8.0, while the solubility steeply rises both at higher and lower pH.
This type of phosphate solubilization can be applicable to a wide range of other redox-active metals, such as Mn2+/3+/4+-containing phosphate minerals (Schwab 1989), as well as phosphates chemisorbed to oxyhydroxides of many other redox-active metals, such as phosphate-containing ferric oxides/oxyhydroxides.
-
4.
Enzymatically driven dissolution of phosphate may be an essential process in organic phosphorus-rich soils, involving phosphatase-driven hydrolysis of poorly soluble organic phosphate esters, which release inorganic phosphate (Rodriguez et al. 2006; Nannipieri et al. 2011). However, in the presence of Al3+ and Fe3+, enzymatic release of phosphate from phytate by incubation with a fungal phytase was affected. This resulted from formation of insoluble Al3+ or Fe3+ phytate complexes, rather than precipitation of soluble orthophosphate after its enzymatically driven release from phytate (He et al. 2006).
-
5.
Indirect solubilization, such as microbial stimulation of exudation of organic acids by plants (Arcand and Schneider 2006), by its mechanism is close to type 2 solubilization (listed above). However, it is directly related to plant–microbe interactions in the rhizosphere and thus is relevant to rock phosphate solubilization in extensively planted agriculture soils (see also Table 2).
Sometimes, in the PSB-related literature, contradictory results are found. For example, when inoculation of plants with phosphate-solubilizing bacteria occurs, increased uptake of P from soil (Kucey et al. 1989), the phosphate-solubilizing activity of the strains studied by Belimov et al. (2002) is unimportant in P uptake by inoculated plants.
The influence of coexisting carbonates on mineral phosphate solubilization
If there are suitable conditions for saturation of an aqueous medium with carbon dioxide (CO2), it might facilitate dissolution of calcium phosphate minerals (see general Eq. (1)), since it can lower the pH to 3.8, which is the pH of a saturated CO2 solution at room temperature (Gerretsen 1948). This results from the equilibrium with partial formation of a very weak carbonic acid that weakly dissociates, according to Eq. (7):
Nevertheless, in the presence of coexisting mineral carbonates, microbially driven mineral phosphate solubilization may be significantly retarded. This particularly relates to the phosphate solubilization mechanisms involving acidification of the medium, for example, antiport of protons (H+) in the course of NH +4 assimilation or release of organic/inorganic acids. In this case, a parallel reaction involves the carbonate-containing phase, such as for calcium carbonate:
In this case, as what follows from the chemistry of heterogeneous processes, formation of the gaseous phase (CO2) and its removal from the reaction medium (volatilization) shifts the equilibrium toward carbonate dissolution, rather than possible parallel solubilization of the coexisting poorly soluble mineral phosphate. In this case, the consumption of protons in Eq. (8) prevents acidification of the medium and, correspondingly, retards mineral phosphate solubilization. Moreover, the appearing excess of calcium ions released from the dissolving calcium carbonate in Eq. (8) would keep mineral phosphate components within the solid phase by retarding reaction in Eq. (4), in accordance with the mass action law and/or binding the organic acid anions in a complex, such as that formed in Eq. (5), but without phosphate dissolution, as follows:
Corresponding experimental evidence was already reported at the beginning of the twentieth century. In the course of microbial release of acids, in soils rich in calcium carbonate, there would be only small quantities of phosphorus liberated (Kelley 1912; cited in Greaves 1922).
However, according to the experimental data of Szymkiewicz-Dabrowska et al. (2002), adding well-soluble ammonium or potassium bicarbonates (NH4HCO3, KHCO3) or carbonates ((NH4)2CO3, K2CO3) to soils mixed with AlPO4·2H2O, FePO4·2H2O, and Ca3(PO4)2 resulted in increasing solubilities of the latter three minerals within a few days. This could be attributed to slowly ongoing hydrolysis of the phosphates with the formation of basic salts, after the pH is raised in the presence of HCO −3 and, especially, CO 2−3 anions owing to their hydrolysis. Specifically, surface hydrolysis reactions for Al (variscite) and FeIII (strengite) phosphates occurring at higher pH values, releasing phosphate ions into solution and forming more basic insoluble metal phosphates, were described earlier (Bache 1963). Thus, as a result, a part of the phosphate anions in a mineral are substituted by OH− ions within the solid phase and therefore become solubilized.
Overall discussion
Although the literature contains many reports on the use of TCP as a universal selection factor for PSB, empirical reports show that most of the strains isolated using this selector failed to deliver. Consequently, they were discarded and forgotten. Analysis of the literature and chemical considerations show that biological phosphate solubilization is a very complex phenomenon affected by numerous factors where each cannot be evaluated and tested separately. This happens because of the enormous possibilities presented by many soil types, insoluble phosphate species, and many potential PSB residing in these soils. Practically, it is not enough to know which organic acid is produced by the potential PSB and theoretically calculate how it functions to make insoluble P more soluble in the soils. Even though there are less soluble metal-P compounds than TCP, such as fluorapatite and hydroxyapatite, acid solubility knowledge of each compound alone, when tested in conjunction with the common knowledge of the main organic acids produced by plants or by the PSB, is not enough to predict with certainty what specific testing combination (organic acid–metal-P–plant–PSB) should be chosen. Too many, ever changing, chemical and later biological parameters are involved, and such theoretical prediction would not be reliable. Perhaps, this is the main reason why there are so many potential PSB isolated in vitro and such a low number of isolates that proved to be successful in inoculated plants. A practical strategy would be to test each PSB–plant interaction experimentally. Yet this would be feasible only when a very small number of isolates are selected in the first place. Isolation of PSB with TCP that produces numerous candidates is therefore not the best strategy and should be replaced.
Another common mistake prevailing in the literature is the measurement of growth promotion of plants inoculated by PSB in P-deficient soils as an indirect indicator for P solubilization. This assumption is based on the fact that many PGPB that are also PSB are known (Puente et al. 2004a, b, 2009a, b, and more). Yet the effect on plant growth, mostly on plant growth parameters, is not necessarily related to phosphate solubilization, but rather to numerous other plant growth-promoting traits (Barret et al. 2011; Bashan and de-Bashan 2005, 2010; Lugtenberg and Kamilova 2009). A better indication that a potential PSB truly contributes to P content and metabolism of the plants is to evaluate P-related parameters of plant nutrition.
The most common laboratory test for P solubilization is the halo formation test known for half a century (Pikovskaya 1948). Here, the potential PSB grows on solid, rich medium in Petri dishes, where the sole P source is an insoluble P. Once a colony is growing, the solubilization process produces a halo, where the intensity of the solubilization is proportional to the size of the halo (Nautiyal 1999). Yet, many times, potential PSB are growing on these media without producing a visible halo, even after several transfers to the same medium (Puente et al. 2004a, b, 2009a, b; Lopez et al. 2011). This indicates that the importance of a halo, as a sole marker for P solubilization, is largely overestimated and is practically inadequate (see footnote a of Table 2).
Because current biological and chemical knowledge indicates that a universal selection factor for biological phosphate solubilization does not exist, the following conclusions and potential guidelines can be drawn:
-
TCP, as a universal factor for isolating and evaluating PSB, is not a good selector according to much literature concerning failure with inoculated plants when using this compound for selection of PSB. Consequently, its use as sole selector should be abolished and the general technique should be complemented.
-
There are several other common, insoluble metal-P compounds, some more insoluble than TCP, but none can replace it reliably for a universal selection factor because of chemical interactions.
-
A combination of two to three metal-P compounds, when used together or in a tandem should replace the sole TCP as an initial selection factor. These combinations may or may not include TCP in the mix in alkaline soils.
-
The selection of the metal-P candidates for potential PSB will depend on the type of soil (alkaline, acidic, or organic-rich) where the PSB will be used.
-
Production of a halo on a solid agar medium should not be considered the sole test for P solubilization. When colonies grow without a halo after several replacements of the medium, an additional test in liquid media to assay P dissolution should be performed.
-
The few bacterial isolates that are obtained after such rigorous selection should be further tested for abundant production of organic acids.
-
Isolates complying with the above criteria should be tested on a model plant as the ultimate test for potential P solubilization.
-
Parameters related to P nutrition in plants should be tested, not growth promotion in general.
-
Consequently, we propose that new manuscripts that report initial isolation of “potential PSB” should not be considered for publication without exhaustive testing.
References
Ahmad F, Ahmad I, Khan MS (2008) Screening of free-living rhizospheric bacteria for their multiple plant growth promoting activities. Microbiol Res 163:173–181
Ahn PM (1993) Tropical soils and fertilizer use. Intermediate tropical agriculture series. Longman, Essex
Alikhani HA, Saleh-Rastin N, Antoun H (2006) Phosphate solubilization activity of rhizobia native to Iranian soils. Plant Soil 287:35–41
Antoun H, Beauchamp CJ, Goussard N, Chabot R, Lalande R (1998) Potential of Rhizobium and Bradyrhizobium species as plant growth promoting rhizobacteria on non-legumes: effect on radishes (Raphanus sativus L.). Plant Soil 204:57–67
Arcand MM, Schneider KD (2006) Plant- and microbial-based mechanisms to improve the agronomic effectiveness of phosphate rock: a review. An Acad Bras Cienc 78:791–807
Babana AH, Antoun H (2005) Biological system for improving the availability of Tilemsi phosphate rock for wheat (Triticum aestivum L) cultivated in Mali. Nutr Cycl Agroecosyst 72:147–157
Babana AH, Antoun H (2006) Effect of Tilemsi phosphate rock-solubilizing microorganisms on phosphorus uptake and yield of field-grown wheat (Triticum aestivum L) in Mali. Plant Soil 287:51–58
Bache BW (1963) Aluminium and iron phosphate studies relating to soils. I. Solution and hydrolysis of variscite and strengite. J Soil Sci 14:113–123
Baig KS, Arshad M, Shaharoona B, Khalid A, Ahmed I (2012) Comparative effectiveness of Bacillus spp. possessing either dual or single growth-promoting traits for improving phosphorus uptake, growth and yield of wheat (Triticum aestivum L.). Ann Microbiol. doi:10.1007/s13213-011-0352-0
Bardiya MC, Gaur AC (1974) Isolation and screening of microorganisms dissolving low-grade rock phosphate. Folia Microbiol 19:386–389
Barret M, Morrissey JP, O'Gara F (2011) Functional genomics analysis of plant-growth promoting rhizobacterial traits involved in rhizosphere competence. Biol Fertil Soils 47:729–743
Bashan Y, de-Bashan LE (2005) Bacteria/Plant growth-promotion. In: Hillel D (ed) Encyclopedia of soils in the environment. Vol. 1. Elsevier, Oxford, pp 103–115
Bashan Y, de-Bashan LE (2010) How the plant growth-promoting bacterium Azospirillum promotes plant growth—a critical assessment. Adv Agron 108:77–136
Bashan Y, Moreno M, Troyo E (2000) Growth promotion of the seawater-irrigated oil seed halophyte Salicornia bigelovii inoculated with mangrove rhizosphere bacteria and halotolerant Azospirillum spp. Biol Fertil Soils 32:265–272
Bationo A, Ayuk E, Ballo D, Kone M (1997) Agronomic and economic evaluation of Tilemsi phosphate rock in different agroecological zones of Mali. Nutr Cycl Agrosyst 48:179–189
Belimov AA, Safronova VI, Mimura T (2002) Response of spring rape (Brassica napus var. oleifera L.) to inoculation with plant growth promoting rhizobacteria containing 1-aminocyclopropane-1-carboxylate deaminase depends on nutrient status of the plant. Can J Microbiol 48:189–199
Ben Farhat M, Farhat A, Bejar W, Kammoun R, Bouchaala K, Fourati A, Antoun H, Bejar S, Chouayekh H (2009) Characterization of the mineral phosphate solubilizing activity of Serratia marcescens CTM 50650 isolated from the phosphate mine of Gafsa. Arch Microbiol 191:815–824
Brosheer JC, Lenfesty FA, Anderson JF Jr (1954) Solubility in the system aluminum phosphate–phosphoric acid–water. J Am Chem Soc 76:5951–5956
Castagno LN, Estrella MJ, Sannazzaro AI, Grassano AE, Ruiz OA (2011) Phosphate-solubilization mechanism and in vitro plant growth promotion activity mediated by Pantoea eucalypti isolated from Lotus tenuis rhizosphere in the Salado River Basin (Argentina). J Appl Microbiol 110:151–1165
Cawthray GR (2003) An improved reversed-phase liquid chromatographic method for the analysis of low-molecular mass organic acids in plant root exudates. J Chromatogr 1011:233–240
Chabot R, Antoun H, Cescas MP (1996) Growth promotion of maize and lettuce by phosphate-solubilizing Rhizobium leguminosarium biovar phaseoli. Plant Soil 184:311–321
Chang C-H, Yang S-S (2009) Thermo-tolerant phosphate-solubilizing microbes for multi-functional biofertilizer preparation. Bioresour Technol 100:1648–1658
Chen YP, Rekha PD, Arunshen AB, Lai WA, Young CC (2006) Phosphate solubilizing bacteria from subtropical soil and their tricalcium phosphate solubilizing abilities. Appl Soil Ecol 34:33–41
Chung H, Park M, Madhaiyan M, Seshadri S, Song J, Cho H, Sa T (2005) Isolation and characterization of phosphate solubilizing bacteria from the rhizosphere of crop plants of Korea. Soil Biol Biochem 37:1970–1974
Collavino MM, Sansberro PA, Mroginski LA, Aguilar OM (2010) Comparison of in vitro solubilization activity of diverse phosphate-solubilizing bacteria native to acid soil and their ability to promote Phaseolus vulgaris growth. Biol Fertil Soils 46:727–738
de Freitas JR, Banerjee NR, Germida JJ (1997) Phosphate solubilizing rhizobacteria enhance the growth and yield but not phosphorus uptake of canola (Brassica napus L.). Biol Fertil Soils 24:358–364
de-Bashan LE, Bashan Y (2004) Recent advances in removing phosphorus from wastewater and its future use as fertilizer (1997–2003). Water Res 38:4222–4246
Dorozhkin SV (2009) Calcium orthophosphate-based biocomposites and hybrid biomaterials. J Mater Sci 44:2343–2387
Dorozhkin SV (2011) Calcium orthophosphates. Occurrence, properties, biomineralization, pathological calcification and biomimetic applications. Biomatter 1:121–164
Duffield JR, Edwards K, Evans DA, Morrish DM, Vobe RA, Williams DR (1991) Low molecular mass aluminum complex speciation in biofluids. J Coord Chem 23:277–290
El-Tarabily KA, Youssef T (2010) Enhancement of morphological, anatomical and physiological characteristics of seedlings of the mangrove Avicennia marina inoculated with a native phosphate-solubilizing isolate of Oceanobacillus picturae under greenhouse conditions. Plant Soil 332:147–162
Evans WG, Pierce AG (1981) Calcium-phytate complex formation studies. J Am Oil Chem Soc 58:850–851
Fankem H, Ngo Nkot L, Deubel A, Quinn J, Merbach W, Etoa F-X, Nwaga D (2008) Solubilization of inorganic phosphates and plant growth promotion by strains of Pseudomonas fluorescens isolated from acidic soils of Cameroon. Afr J Microbiol Res 2:171–178
Fernández LA, Zalba P, Gómez MA, Sagardoy MA (2007) Phosphate-solubilization activity of bacterial strains in soil and their effect on soybean growth under greenhouse conditions. Biol Fertil Soils 43:805–809
Gerretsen FC (1948) The influence of microorganisms on the phosphate intake by the plant. Plant Soil 1:51–81
Ghassemi M, Recht HL (1971) Phosphate precipitation with ferrous iron. Project No. 17010 EKI. US Environmental Protection Agency, Washington, DC, 69 pp
Gillis MB, Edwards HM Jr, Young RJ (1962) Studies on the availability of calcium orthophosphates to chickens and turkeys. J Nutr 78:155–161
Goldstein AH (1995) Recent progress in understanding the molecular genetics and biochemistry of calcium phosphate solubilization by gram negative bacteria. Biol Agric Hortic 12:185–193
Goldstein AH (2007) Future trends in research on microbial phosphate solubilization: one hundred years of insolubility. In: Velázquez E, Rodríguez-Barrueco C (eds) First international meeting on microbial phosphate solubilization. Developments in plant and soil sciences, Vol. 102. Springer, Dordrecht, pp 91–96
Goldstein AH, Krishnaraj PU (2007) Phosphate solubilizing microorganisms vs. phosphate mobilizing microorganisms: what separates a phenotype from a trait? In: Velázquez E, Rodríguez-Barrueco C (eds) First international meeting on microbial phosphate solubilization. Developments in plant and soil sciences, vol. 102. Springer, Dordrecht, pp 203–213
Goldstein AH, Liu ST (1987) Molecular cloning and regulation of a mineral phosphate solubilizing gene from Erwinia herbicola. Biotechnology 5:72–74
Goldstein AH, Braverman K, Osorio N (1999) Evidence for mutualism between a plant growing in a phosphate-limited desert environment and a mineral phosphate solubilizing (MPS) rhizobacterium. FEMS Microbiol Ecol 30:295–300
Greaves JE (1922) Influence of salts on bacterial activities of soil. Bot Gaz 73:161–180
Grynspan F, Cheryan M (1983) Calcium phytate: effect of pH and molar ratio on in vitro solubility. J Am Oil Chem Soc 60:1761–1764
Gulati A, Rahi P, Vyas P (2008) Characterization of phosphate-solubilizing fluorescent pseudomonads from the rhizosphere of seabuckthorn growing in the cold deserts of Himalayas. Curr Microbiol 56:73–79
Gulati A, Sharma N, Vyas P, Sood S, Rahi P, Pathania V, Prasad R (2010) Organic acid production and plant growth promotion as a function of phosphate solubilization by Acinetobacter rhizosphaerae strain BIHB 723 isolated from the cold deserts of the trans-Himalayas. Arch Microbiol 192:975–983
Gyaneshwar P, Kumar GN, Parekh LJ, Poole PS (2002) Role of soil microorganisms in improving P nutrition of plants. Plant Soil 245:83–93
Hameeda B, Harini G, Rupela OP, Wani SP, Reddy G (2008) Growth promotion of maize by phosphate solubilizing bacteria isolated from composts and macrofauna. Microbiol Res 163:234–242
Harris JN, New PB, Martin PM (2006) Laboratory tests can predict beneficial effects of phosphate-solubilising bacteria on plants. Soil Biol Biochem 38:1521–1526
Haynes RJ (1982) Effects of liming on phosphate availability in acid soils. Plant Soil 68:289–308
He Z, Ohno T, Cade-Menun BJ, Erich MS, Honeycutt CW (2006) Spectral and chemical characterization of phosphates associated with humic substances. Soil Sci Soc Am J 70:1741–1751
Hill JE, Kysela D, Elimelech M (2007) Isolation and assessment of phytate-hydrolysing bacteria from the DelMarVa Peninsula. Environ Microbiol 9:3100–3107
Hoffland E (1992) Quantitative evaluation of the role of organic acid exudation in the mobilization of rock phosphate by rape. Plant Soil 140:279–289
Idriss EE, Makarewicz O, Farouk A, Rosner K, Greiner R, Bochow H, Richter T, Borriss R (2002) Extracellular phytase activity of Bacillus amyloliquefaciens FZB45 contributes to its plant-growth-promoting effect. Microbiology 148:2097–2109
Illmer P, Schinner F (1995) Solubilization of inorganic calcium phosphates-solubilization mechanisms. Soil Biol Biochem 27:257–263
Illmer P, Barbato A, Schinner F (1995) Solubilization of hardly-soluble AlPO4 with P-solubilizing microorganisms. Soil Biol Biochem 27:265–270
Iqbal U, Jamil N, Ali I, Hasnain S (2010) Effect of zinc-phosphate-solubilizing bacterial isolates on growth of Vigna radiate. Ann Microbiol 60:243–248
Ishio S, Kuwahara M, Nakawaga H (1986) Conversion of AlPO4-P to Fe-bound P in sea sediments. B Jpn Soc Sci Fish 52:901–911
Jiang J-Q, Graham NJD (1998) Pre-polymerised inorganic coagulants and phosphorus removal by coagulation—a review. Water SA 24:237–244
Johri JK, Surange S, Nautiyal CS (1999) Occurrence of salt, pH, and temperature-tolerant, phosphate-solubilizing bacteria in alkaline soils. Curr Microbiol 39:89–93
Jones DL (1998) Organic acids in the rhizosphere—a critical review. Plant Soil 205:25–44
Jones DL, Darrah PR (1994) Role of root derived organic acids in the mobilization of nutrients from the rhizosphere. Plant Soil 166:247–257
Jorquera MA, Hernandez MT, Rengel Z, Marschner P, Mora ML (2008) Isolation of culturable phosphobacteria with both phytate-mineralization and phosphate-solubilization activity from the rhizosphere of plants grown in a volcanic soil. Biol Fertil Soils 44:1025–1034
Katiyar V, Goel R (2003) Solubilization of inorganic phosphate and plant growth promotion by cold tolerant mutants of Pseudomonas fluorescens. Microbiol Res 158:163–168
Kelley WP (1912) The effects of calcium and magnesium carbonates on some biological transformations of nitrogen in soils. Univ Calif Publ Agric Sci 1:39–49
Kim KY, McDonald GA, Jordan D (1997) Solubilization of hydroxypatite by Enterobacter agglomerans and cloned Escherichia coli in culture medium. Biol Fertil Soils 24:347–352
Kim KY, Jordan D, Krishnan HB (1998) Expression of genes from Rahnella aquatilis that are necessary for mineral phosphate solubilization in Escherichia coli. FEMS Microb Lett 159:121–127
Kpomblekou AK, Tabatabai MA (1994) Effect of organic acids on release of phosphorus from phosphate rocks. Soil Sci 158:442–453
Kucey RMN, Janzen HH, Leggett ME (1989) Microbially mediated increases in plant-available phosphorus. Adv Agron 42:199–228
Kumar V, Narula N (1999) Solubilization of inorganic phosphates and growth emergence of wheat as affected by Azotobacter chroococcum mutants. Biol Fertil Soils 28:301–305
Linu MS, Stephen J, Jisha MS (2009) Phosphate solubilizing Gluconacetobacter sp., Burkholderia sp. and their potential interaction with cowpea (Vigna unguiculata (L.) Walp.). Int J Agri Res 4:79–87
Liu H, Wu XQ, Ren JH, Ye JR (2011) Isolation and identification of phosphobacteria in poplar rhizosphere from different regions of China. Pedosphere 21:90–97
Lobartini JC, Tan KH, Pape C (1998) Dissolution of aluminum and iron phosphate by humic acids. Commun Soil Sci Plant Anal 29:535–544
Lopez BR, Bashan Y, Bacilio M (2011) Endophytic bacteria of Mammillaria fraileana, an endemic rock-colonizing cactus of the Southern Sonoran Desert. Arch Microbiol 193:527–541
Lopez BR, Tinoco-Ojanguren C, Bacilio M, Mendoza A, Bashan Y (2012) Endophytic bacteria of the rock-dwelling cactus Mammillaria fraileana affect plant growth and mobilization of elements from rocks. Environ Exp Bot 81:26–36
Lugtenberg B, Kamilova F (2009) Plant-growth-promoting rhizobacteria. Annu Rev Microbiol 63:541–556
Mamta Rahi P, Pathania V, Gulati A, Singh B, Bhanwra RK, Tewari R (2010) Stimulatory effect of phosphate-solubilizing bacteria on plant growth, stevioside and rebaudioside-A contents of Stevia rebaudiana Bertoni. Appl Soil Ecol 46:222–229
Martin RB (1997) The importance of aluminium chemistry for biological systems. In: Zatta PF, Alfrey AC (eds) Aluminium toxicity in infants’ health and disease. World Scientific, Singapore, pp 3–15
Mehta S, Nautiyal CS (2001) An efficient method for qualitative screening of phosphate-solubilizing bacteria. Curr Microbiol 43:51–56
Merbach W, Fankem H, Deubel A (2009) Influence of rhizosphere bacteria of African oil palm (Elaeis guineensis) on calcium, iron, and aluminum phosphate in vitro mobilization. In: International symposium “Root Research and Applications”, 2–4 September 2009. BOKU, Vienna, Austria. URL: http://asrr.boku.ac.at/fileadmin/files/RRcd/session03/poster/042.pdf
Merbach W, Deubel A, Gransee A, Ruppel S, Klamroth A-K (2010) Phosphorus solubilization in the rhizosphere and its possible importance to determine phosphate plant availability in soil. A review with main emphasis on German results. Arch Agron Soil Sci 56(2):119–138
Middleton VG (ed) (2003) Encyclopedia of sediments and sedimentary rocks. Encyclopedia of earth sciences series. Kluwer, Dordrecht
Nannipieri P, Giagnoni L, Landi L, Renella G (2011) Role of phosphatase enzymes in soil. In: Bunemann EK, Oberson A, Frossard E (eds) Phosphorus in action. Soil biology vol. 26. Springer, Berlin, pp 215–241
Nautiyal CS (1999) An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol Lett 170:265–270
Naz I, Bano A (2010) Biochemical, molecular characterization and growth promoting effects of phosphate solubilizing Pseudomonas sp. isolated from weeds grown in salt range of Pakistan. Plant Soil 334:199–207
Ogut M, Er F, Kandemir N (2010) Phosphate solubilization potentials of soil Acinetobacter strains. Biol Fertil Soils 46:707–715
Oliveira CA, Alves VMC, Marriel IE, Gomes EA, Scotti MR, Carneiro NP, Guimarães CT, Schaffert RE, Sà NMH (2009) Phosphate solubilizing microorganisms isolated from rhizosphere of maize cultivated in an oxisol of the Brazilian Cerrado Biome. Soil Biol Biochem 4:1782–1787
Park K-H, Lee O-M, Jung H-I, Jeong J-H, Jeon Y-D, Hwang D-Y, Lee C-Y, Son H-J (2010) Rapid solubilization of insoluble phosphate by a novel environmental stress-tolerant Burkholderia vietnamiensis M6 isolated from ginseng rhizospheric soil. Appl Microbiol Biotechnol 86:947–955
Peix A, Rivas R, Mateos PF, Martinez-Molina E, Rodriguez-Barrueco C, Velazquez E (2003) Pseudomonas rhizosphaerae sp. nov., a novel species that actively solubilizes phosphate in vitro. Int J Syst Evol Microbiol 53:2067–2072
Peix A, Rivas R, Santa-Regina I, Mateos PF, Martinez-Molina E, Rodriguez-Barrueco C, Velazquez E (2004) Pseudomonas lutea sp. nov., a novel phosphate-solubilizing bacterium isolated from the rhizosphere of grasses. Int J Syst Evol Microbiol 54:847–850
Pérez E, Sulbarán M, Ball MM, Yarzábal LA (2007) Isolation and characterization of mineral phosphate-solubilizing bacteria naturally colonizing a limonitic crust in the south-eastern Venezuelan region. Soil Biol Biochem 39:2905–2914
Pikovskaya RI (1948) Mobilization of phosphates in soil in relation with vital activity of some microbial species. Mikrobiologiya 17:362–370 (in Russian)
Puente ME, Bashan Y, Li CY, Lebsky VK (2004a) Microbial populations and activities in the rhizoplane of rock-weathering desert plants. I. Root colonization and weathering of igneous rocks. Plant Biol 6:629–642
Puente ME, Li CY, Bashan Y (2004b) Microbial populations and activities in the rhizoplane of rock-weathering desert plants. II. Growth promotion of cactus seedlings. Plant Biol 6:643–650
Puente ME, Li CY, Bashan Y (2009a) Rock-degrading endophytic bacteria in cacti. Environ Exp Bot 66:389–401
Puente ME, Li CY, Bashan Y (2009b) Endophytic bacteria in cacti seeds can improve the development of cactus seedlings. Environ Exp Bot 66:402–408
Rajan SSS, Watkinson JH, Sinclair AG (1996) Phosphate rocks for direct application to soils. Adv Agron 57:77–159
Rajapaksha RMCP, Herath D, Senanayake AP, Senevirathne MGTL (2011) Mobilization of rock phosphate phosphorus through bacterial inoculants to enhance growth and yield of wetland rice. Commun Soil Sci Plant Anal 42:301–314
Rajkumar M, Nagendran R, Lee KJ, Lee WH, Kim SZ (2006) Influence of plant growth promoting bacteria and Cr6+ on the growth of Indian mustard. Chemosphere 62:741–748
Rengel Z, Marschner P (2005) Nutrient availability and management in the rhizosphere: exploiting genotypic differences. New Phytol 168:305–312
Reyes I, Baziramakenga R, Bernier L, Antoun H (2001) Solubilization of phosphate rocks and minerals by a wild-type strain and two UV-induced mutants of Penicillium rugulosum. Soil Biol Biochem 33:1741–1746
Reyes I, Valery A, Valduz Z (2006) Phosphate-solubilizing microorganisms isolated from rhizospheric and bulk soils of colonizer plants at an abandoned rock phosphate mine. Plant Soil 287:69–75
Richardson A (1994) Soil microorganisms and phosphorus availability. In: Pankhurst CE, Doube BM, Gupta VVSR (eds) Soil biota: management in sustainable farming systems. CSIRO, Victoria, pp 50–62
Richardson A (2001) Prospect for using soil microorganisms to improve the acquisition of phosphorous by plants. Aust J Plant Physiol 28:897–906
Rodriguez H, Fraga R (1999) Phosphate solubilizing bacteria and their role in plant growth promotion. Biotechnol Adv 17:319–339
Rodriguez H, Fraga R, Gonzalez T, Bashan Y (2006) Genetics of phosphate solubilization and its potential applications for improving plant growth-promoting bacteria. Plant Soil 287:15–21
Sackett WG, Patten AJ, Brown CV (1908) The solvent action of soil bacteria upon the insoluble phosphates of raw bonemeal and natural raw rock phosphate. Centralbl Bakteriol 202:688–703
Schwab AP (1989) Manganese-phosphate solubility relationships in an acid soil. Soil Sci Soc Am J 53:1654–1660
Selvakumar G, Joshi P, Nazim S, Mishra PK, Bisht JK, Gupta HS (2009) Phosphate solubilization and growth promotion by Pseudomonas fragi CS11RH1 (MTCC 8984), a psychrotolerant bacterium isolated from a high altitude Himalayan rhizosphere. Biologia 64:239–245
Son H-J, Park G-T, Cha M-S, Heo M-S (2006) Solubilization of insoluble inorganic phosphates by a novel salt- and pH-tolerant Pantoea agglomerans R-42 isolated from soybean rhizosphere. Bioresour Technol 97:204–210
Song O-R, Lee S-J, Lee Y-S, Lee S-C, Kim K-K, Choi Y-L (2008) Solubilization of insoluble inorganic phosphate by Burkholderia cepacia DA23 isolated from cultivated soil. Braz J Microbiol 39:151–156
Srinivasan R, Alagawadi AR, Yandigeri MS, Meena KK, Saxena AK (2012) Characterization of phosphate-solubilizing microorganisms from salt-affected soils of India and their effect on growth of sorghum plants [Sorghum bicolor (L.) Moench]. Ann Microbiol 62:93–105
Stumm W, Morgan JJ (1996) Aquatic chemistry, 3rd edn. Wiley, New York
Szymkiewicz-Dabrowska D, Lachacz A, Huszcza-Ciolkowska G (2002) The contribution of soil solution CO2 (HCO −3 ) to incorporation of sparingly soluble phosphates to the pool of phosphates available for plants. Zeszyty Problemowe Postepow Nauk Rolniczych, no. 484, pp. 701-709 (URL: http://psjc.icm.edu.pl/psjc/cgi-bin/getdoc.cgi?AAAA00721)
Taurian T, Anzuay MS, Angelini JG, Tonelli ML, Ludueña L, Pena D, Ibáñez F, Fabra A (2010) Phosphate-solubilizing peanut associated bacteria: screening for plant growth-promoting activities. Plant Soil 329:421–431
Trivedi P, Sa T (2008) Pseudomonas corrugata (NRRL B-30409) mutants increased phosphate solubilization, organic acid production, and plant growth at lower temperatures. Curr Microbiol 56:140–144
Unno Y, Okubo K, Wasaki J, Shinano T, Osaki M (2005) Plant growth promotion abilities and microscale bacterial dynamics in the rhizosphere of lupin analysed by phytate utilization ability. Environ Microbiol 7:396–404
Vassilev N, Toro M, Vassileva M, Azcon R, Barea JM (1997) Rock phosphate solubilization by immobilized cells of Enterobacter sp. in fermentation and soil conditions. Bioresour Technol 61:29–32
Vazquez P, Holguin G, Puente ME, Lopez-Cortes A, Bashan Y (2000) Phosphate-solubilizing microorganisms associated with the rhizosphere of mangroves in a semiarid coastal lagoon. Biol Fertil Soils 30:460–468
Viruel E, Lucca ME, Siñeriz F (2011) Plant growth promotion traits of phosphobacteria isolated from Puna, Argentina. Arch Microbiol 193:489–496
Wang L, Nancollas GH (2008) Calcium orthophosphates: crystallization and dissolution. Chem Rev 108:4628–4669
Welch SA, Taunton AE, Banfield JF (2002) Effect of microorganisms and microbial metabolites on apatite dissolution. Geomicrobiol J 19:343–367
Whitelaw MA (1999) Growth promotion of plants inoculated with phosphate-solubilizing fungi. Adv Agron 69:99–151
Xiang W-L, Liang H-Z, Liu S, Luo F, Tang J, Li M-Y, Che Z-M (2011) Isolation and performance evaluation of halotolerant phosphate solubilizing bacteria from the rhizospheric soils of historic Dagong brine well in China. World J Microbiol Biotechnol 27:2629–2637
Xiao CQ, Chi RA, Li WS, Zheng Y (2011) Biosolubilization of phosphorus from rock phosphate by moderately thermophilic and mesophilic bacteria. Miner Eng 24:956–958
Yu X, Liu X, Zhu TH, Liu GH, Mao C (2011) Isolation and characterization of phosphate-solubilizing bacteria from walnut and their effect on growth and phosphorus mobilization. Biol Fertil Soils 47:437–446
Zou K, Binkley D, Doxtader KG (1992) A new method for estimating gross phosphorus mineralization and immobilization rates in soils. Plant Soil 147:243–250
Acknowledgments
We thank Prof. Hani Antoun from Laval University, Quebec, Canada for proposing the type of evaluations that were needed for this essay. Ira Fogel of CIBNOR provided editorial services. Preparation of this essay was supported by The Bashan Foundation, USA.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study is dedicated to the memory of the German/Spanish mycorrhizae researcher Dr. Horst Vierheilig (1964–2011) of CSIC, Spain.
Rights and permissions
About this article
Cite this article
Bashan, Y., Kamnev, A.A. & de-Bashan, L.E. Tricalcium phosphate is inappropriate as a universal selection factor for isolating and testing phosphate-solubilizing bacteria that enhance plant growth: a proposal for an alternative procedure. Biol Fertil Soils 49, 465–479 (2013). https://doi.org/10.1007/s00374-012-0737-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-012-0737-7