Abstract
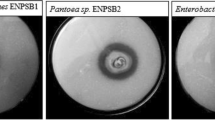
Many phosphate solubilizing microorganisms (PSM) require external pyrroloquinoline quinone (PQQ) for strong phosphorus (P) solubilization in vitro. The objective of this study was to isolate efficient and PQQ-independent PSM. A total of 21 PSM were isolated from the rhizosphere soil of wheat and maize grown in the pots. Acinetobacter strains were the only PQQ-independent and most effective solubilizers of tricalcium phosphate containing agar. The mean P dissolved in liquid cultures of Acinetobacter strains in a 5-day incubation ranged from 167 to 888 μg/ml P. The pH dropped to below 4.7 from 7.8 in six isolates, which produced gluconic acid in concentrations ranging between 27.5 and 37.5 mM. There was a linear regression between soluble P and gluconic acid concentrations in the bacterial cultures (P < 0.05; R 2 = 0.59). Inoculation with Acinetobacter sp. WR922 significantly (P < 0.05) increased wheat (Triticum aestivum L.) P content by 27% at 15 days after emergence (DAE) and dry matter by 15% at 30 DAE compared to the control. The plant P content in inoculated plants at 30 DAE was linearly correlated with soluble P of the bacterial cultures (P < 0.05; R 2 = 0.69). Gluconic acid production directly affected phosphate solubilization in vitro, which in turn influenced plant P content of inoculated plants in PQQ-independent P-solubilizing Acinetobacter strains.



Similar content being viewed by others
References
Adamowicz M, Conway T, Nickerson KW (1991) Nutritional complementation of oxidative glucose metabolism in Escherichia coli via pyrroloquinoline quinone-dependent glucose dehydrogenase and the Entner–Doudoroff pathway. Appl Environ Microbiol 57:2012–2015
Babu-Khan S, Yeo TC, Martin WL, Duron MR, Rogers RD, Goldstein AH (1995) Cloning of a mineral phosphate-solubilizing gene from Pseudomonas cepacia. Appl Environ Microbiol 61:972–978
Baldani VLD, Döbereiner J (1980) Host plant specificity in the infection of cereals with Azospirilum spp. Appl Environ Microbiol 12:433–439
Bashan Y, de-Bashan LE (2005) Bacteria/plant growth-promotion. In: Hillel D (ed) Encyclopedia of soils in the environment, vol 1. Elsevier, Oxford, pp 103–115
Baumann P (1968) Isolation of Acinetobacter from soil and water. J Bacteriol 96:39–42
Chabot R, Beauchamp CJ, Kloepper JW, Antoun H (1998) Effect of phosphorus on root colonization and growth promotion of maize by bioluminescent mutants of phosphate-solubilizing Rhizobium leguminosarum biovar phaseoli. Soil Biol Biochem 12:1615–1618
Chung H, Park M, Madhaiyan M, Seshadri S, Song J, Cho H, Sa T (2005) Isolation and characterization of phosphate solubilizing bacteria from the rhizosphere of crop plants of Korea. Soil Biol Biochem 37:1970–1974
Day PR (1965) Particle fractionation and particle-size analysis. In: Black CA (ed) Methods of soil analysis, part I. American Society of Agronomy, Madison, WI, pp 547–565
de-Bashan LE, Bashan Y (2004) Recent advances in removing phosphorus from wastewater and its future use as fertilizer (1997–2003). Water Res 38:4222–4246
Fliege R, Tong S, Shibata A, Nickerson KW, Conway T (1992) The Entner–Doudoroff pathway in Escherichia coli is induced for oxidative glucose metabolism via pyrroloquinoline quinine-dependent glucose dehydrogenase. Appl Environ Microbiol 58:3826–3829
Gaind S, Gaur AC (2002) Impact of fly ash and phosphate solubilizing bacteria on soybean productivity. Bioresour Technol 85:313–315
Goldstein AH, Braverman K, Osorio N (1999) Evidence for mutualism between a plant growing in a phosphate-limited desert environment and a mineral phosphate solubilizing (MPS) rhizobacterium. FEMS Microbiol Ecol 30:295–300
Goldstein AH, Rogers RD, Mead G (1993) Separating phosphate from ores via bioprocessing. Nat Biotechnol 11:1250–1254
Goosen N, Horsman HP, Huinen RG, van de Putte P (1989) Acinetobacter calcoaceticus genes involved in biosynthesis of the coenzyme pyrrolo-quinoline-quinone: nucleotide sequence and expression in Escherichia coli K-12. J Bacteriol 171:447–455
Guinazu LB, Andres JA, MF Del papa, Pistorio M, Rosas SB (2010) Response of alfalfa (Medicago sativa L) to single and mixed inoculation with phosphate-solubilizing bacteria and Sinorhizobium meliloti. Biol Fertil Soils 46:185–190
Gyaneshwar P, Parekh LJ, Archana G, Poole PS, Collins MD, Hutson RA, Kumar GN (1999) Involvement of a phosphate starvation inducible glucose dehydrogenase in soil phosphate solubilization by Enterobacter asburiae. FEMS Microbiol Lett 171:223–229
Hommes RWJ, Postma PW, Neijssel OM, Tempest DW, Dokter P, Duine JA (1984) Evidence of a quinoprotein glucose dehydrogenase apoenzyme in several strains of Escherichia coli. FEMS Microbiol Lett 24:329–333
Illmer P, Schinner F (1992) Solubilization of inorganic phosphates by microorganisms isolated from forest soil. Soil Biol Biochem 24:389–395
Kim KY, Jordan D, Krishnan HB (1997) Rahnella aquatilis, a bacterium isolated from soybean rhizosphere, can solubilize hydroxyapatite. FEMS Microbiol Lett 153:273–277
Krishnaraj PU, Goldstein AH (2001) Cloning of Serratia marcescens DNA fragment that induces quinoprotein glucose dehydrogenase-mediated gluconic acid production in Escherichia coli in the presence of stationary phase Serratia marcescens. FEMS Microbiol Lett 205:215–220
Kumar V, Narula N (1999) Solubilization of inorganic phosphates and growth emergence of wheat as affected by Azotobacter chroococcum mutants. Biol Fertil Soils 28:301–305
Lin T, Huang H, Shen F, Young C (2006) The protons of gluconic acid are the major factor responsible for the dissolution of tricalcium phosphate by Burkholderia cepacia CC-A174. Bioresour Technol 97:957–960
Lindsey WL, Norwell WA (1978) Development of DTPA soil test for zinc, iron, manganese, and copper. Soil Sci Soc Am J 42:421–428
Liu S, Lee L, Tai C, Hung C, Chang Y, Wolfram JH, Rogers R, Goldstein AH (1992) Cloning of an Erwinia herbicola gene necessary for gluconic acid production and enhanced mineral phosphate solubilization in E. coli HB101: nucleotide sequence and probable involvement in biosynthesis of the co-enzyme pyrroloquinnoline quinone. J Bacteriol 174:5814–5819
Narula N, Kumar V, Behl RK, Deubel A, Gransee A, Merbach W (2000) Effect of P- solubilizing Azotobacter chroococcum on N, P, K uptake in P-responsive wheat genotypes grown under greenhouse conditions. J Plant Nutr Soil Sci 163:393–398
Nautiyal CS, Bhadauria S, Kumar P, Lal H, Mondal R, Verma D (2000) Stress induced phosphate solubilization in bacteria isolated from alkaline soils. FEMS Microbiol Lett 182:291–296
Nelson RE (1982) Carbonate and gypsum. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis, part II: chemical and microbiological properties. SSSA Inc., Madison, WI, pp 181–196
Nelson DW, Sommers LE (1982) Total carbon, organic carbon, and organic matter. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis, part II: chemical and microbiological properties. SSSA Inc., Madison, WI, pp 539–577
Olsen SR, Sommers LE (1982) Phosphorus. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis, part II: chemical and microbiological properties. SSSA Inc., Madison, WI, pp 403–430
Peix A, Mateos PF, Rodriguez-Barrueco C, Martinez-Molina E, Velazquez E (2001) Growth promotion of common bean (Phaseolus vulgaris L.) by a strain of Burkholderia cepacia under growth chamber conditions. Soil Biol Biochem 33:1927–1935
Perez E, Sulbaran M, Ball MM, Yarzabal LA (2007) Isolation and characterization of mineral phosphate-solubilizing bacteria naturally colonizing a limonitic crust in the south-eastern Venezuelan region. Soil Biol Biochem 39:2905–2914
Reyes I, Bernier L, Simard RR, Tanguay P, Antoun H (1999) Characteristics of phosphate solubilization by an isolate of tropical Penicillium rugulosum and two UV-induced mutants. FEMS Microbiol Ecol 28:291–295
Rodriguez H, Fraga R, Gonzales T, Bashan Y (2006) Genetics of phosphate solubilization and its applications for improving plant growth-promoting bacteria. Plant Soil 287:15–21
Son H, Park G, Cha M, Heo M (2006) Solubilization of insoluble inorganic phosphates by a novel salt- and pH-tolerant Pantoea agglomerans R-42 isolated from soybean rhizosphere. Bioresour Technol 97:204–210
Stevenson FJ (1986) Cycles of carbon, nitrogen, phosphorus, sulphur, and micronutrients. Wiley, New York
Tisdale SL, Nelson WL, Beaton JD, Havlin JL (1993) Soil fertility and fertilizers. Macmillian Publishing Co, New York
Towner K (2006) The genus Acinetobacter. In: Dworkin M, Falkow S, Rosenberg E, Schleifer K, Stackebrandt E (eds) The Procaryotes, a handbook on the biology of the bacteria: Proteobacteria, gamma subclass, Volume 6. Springer Science+Business Media, LLC, New York, NY, pp 746–758
van Kleef MAG, Duine JA (1989) Factors relevant in bacterial pyrroloquinoline quinine production. Appl Environ Microbiol 55:1209–1213
van Schie BJ, van Dijken JP, Kuenen JG (1984) Non-coordinated synthesis of glucose dehydrogenase and its prosthetic group PQQ in Acinetobacter and Pseudomonas species. FEMS Microbiol Lett 24:133–138
Vasquez P, Holguin G, Puente ME, Lopez-Cortes A, Bashan Y (2000) Phosphate-solubilizing microorganisms associated with the rhizosphere of mangroves in a semiarid coastal lagoon. Biol Fertil Soils 30:460–468
Acknowledgment
We gratefully acknowledge the funding of this project by the Gaziosmanpaşa and Selçuk Universities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ogut, M., Er, F. & Kandemir, N. Phosphate solubilization potentials of soil Acinetobacter strains. Biol Fertil Soils 46, 707–715 (2010). https://doi.org/10.1007/s00374-010-0475-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00374-010-0475-7