Abstract
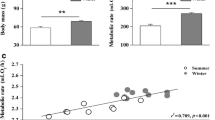
Black-legged kittiwakes (BLKIs) reduce self-maintenance cost through reductions in mass-specific basal metabolic rate (BMR), body mass and the size of visceral organs during the chick-rearing period. In the present study, we measured kidney in vitro oxygen consumption and plasma 3,3′,5-triiodo-l-thyronine (T3) levels of incubating and chick-rearing female BLKIs, to test whether the decrease in BMR is caused mainly by decreased metabolic intensity or simply by reductions in the size of organs with high metabolic intensity. Body mass and body condition were lower in chick-rearing birds compared with the incubating birds. In contrast to the previous findings, however, the kidney mass did not differ between the two breeding stages. Plasma T3 levels decreased substantially during the breeding season, indicating a reduction in BMR. Over the same period, kidney mass-specific oxygen consumption decreased (by 17.2%) from the incubating to the chick-rearing stage. Thus, the reduction in BMR found in breeding BLKIs seems partly explained by adjustments in metabolic intensity of visceral organs. Lowered metabolic intensity of visceral organs would permit increased allocation of energy to offspring at the expense of their own self-maintenance.


Similar content being viewed by others
Abbreviations
- BLKI:
-
Black-legged kittiwake
- BMR:
-
Basal metabolic rate
- CR:
-
Chick rearing
- INC:
-
Incubating
- T3:
-
3,3′,5-triiodo-l-thyronine
- VO2 :
-
Oxygen consumption
References
Barker SB, Klitgaard HM (1952) Metabolism of tissues excised from thyroxin-injected rats. Am J Physiol 170:81–86
Bech C, Langeth I, Gabrielsen GW (1999) Repeatability of basal metabolic rate in breeding female kittiwakes Rissa tridactyla. Proc R Soc Lond B Biol Sci 266:2161–2167
Bech C, Østnes JE (1999) Influence of body composition on the metabolic rate of nestling European shags (Phalacrocorax aristotelis). J Comp Physiol B 169:263–270
Bech C, Langseth I, Moe B, Fyhn M, Gabrielsen GW (2002) The energy economy of the Arctic-breeding kittiwake (Rissa tridactyla): a review. Comp Biochem Physiol A 133:765–770
Barrett RT, Ficler R, Anker-Nilssen T, Rikardsen F (1985) Measurements and weight changes of Norwegian adult Puffins Fratercula arctica and Kittiwakes Rissa tridactyla during the breeding season. Ring Migr 6:102–112
Burness PB, Ydenberg RC, Hochachka PW (1998) Interindividual variability in body composition and resting oxygen consumption rate in breeding tree swallows, Tachycineta bicolour. Physiol Zool 71:247–256
Chappell MA, Bech C, Buttemer WA (1999) The relationship of central and peripheral organ masses to aerobic performance variation in house sparrows. J Exp Biol 202:2269–2279
Chastel O, Lacroix A, Kersten M (2003) Pre-breeding energy requirements: thyroid hormone, metabolism and the timing of reproduction in house sparrows. J Avian Biol 34:298–306
Clausen T, Hardeveld CV, Evert ME (1991) Significance of cation transport in control of energy metabolism and thermogenesis. Physiol Rev 71:733–774
Drent RH, Daan S (1980) The prudent parent—energetic adjustments in avian breeding. Ardea 68:225–252
Fridolfsson AK, Ellegren H (1999) A simple and universal method for molecular sexing of non-ratite birds. J Avian Biol 30:116–121
Goglia F, Silvestri E, Lanni A (2002) Thyroid hormones and mitochondria. Biosci Rep 22:17–32
Golet HG, Irons DB (1999) Raising young reduces body condition and fat stores in black-legged kittiwakes. Oecologia 120:530–538
Ismail-Beigi F, Edelman IS (1971) The mechanism of calorigenic action of thyroid hormone. Stimulation of Na+ + K+-activated adenosinetriphosphatase activity. J Gen Physiol 57:710–722
Klandorf H, Sharp PJ, Duncan IJH (1978) Variations in levels of plasma thyroxine and triiodothyronine in juvenile female chickens during 24- and 16-hr lighting cycles. Gen Comp Endocrinol 36:238–243
Krebs HA (1950) Body size and tissue respiration. Biochim Biophys Acta 4:249–269
Langseth I, Moe B, Fyhn M, Gabrielsen GW, Bech C (2000) Flexibility of basal metabolic rate in Arctic breeding kittiwakes (Rissa tridactyla). In: Heldmaier G, Klingenspor M (eds) Life in the cold. Springer, Berlin, pp 471–477
Lundgren BD, Kiessling KH (1985) Seasonal variation in catabolic enzyme activities in breast muscle of some migratory birds. Oecologia 66:468–471
Lundgren BD, Kiessling KH (1986) Catabolic enzyme activities in pectoralis muscle of premigratory and migratory juvenile Reed Warblers, Acrocephalus scirpaceus (Herm.). Oecologia 68:529–532
McNabb FMA (2000) Thyroids. In: Whittow GC (ed) Avian physiology. Academic Press, San Diego, pp 461–472
Merkt JR, Taylor R (1994) “Metabolic switch” for desert survival. Proc Natl Acad Sci USA 91:12313–12316
Moe B, Langseth I, Fyhn M, Gabrielsen GW, Bech C (2002) Changes in body condition in breeding kittiwakes Rissa tridactyla. J Avian Biol 33:225–234
Moe B, Brunvoll S, Mork D, Brobakk TE, Bech C (2004) Developmental plasticity of physiology and morphology in diet-restricted European shag nestlings (Phalacrocorax aristotelis). J Exp Biol 207:4067–4076
Moe B, Stølevik E, Bech C (2005) Ducklings exhibit substantial energy-saving mechanisms as a response to short-term food shortage. Physiol Biochem Zool 78:90–104
Moreno J (1989) Strategies of mass change in breeding birds. Biol J Linn Soc 37:297–310
O’Connor TP (1995) Seasonal acclimatization of lipid mobilization and catabolism in house-finches (Carpodacus maxicanus). Physiol Zool 68:985–1005
Piersma T (2002) Energetic bottlenecks and other design constrains in avian annual cycles. Integ Comp Biol 42:51–67
Piersma T, Lindstrøm Å (1997) Rapid reversible changes in organ size as a component of adaptive behaviour. Trends Ecol Evol 12:134–138
Piersma T, Gessaman JA, Dekinga A, Visser H (2004) Gizzard and other mass components increase, yet basal metabolic rate decrease, when red knots Calidris canutus are shifted from soft to hard-shelled food. J Avian Biol 35:99–104
Ricklefs RE (1974) Energetics of reproduction in birds. In: Paynter RA (ed) Avian energetics Publ Nuttal Ornithol Club No 15. Cambridge, MA, pp 152–297
Roff DA (1992) The evolution of life histories–theory and analysis. Routledge, Chapman & Hall, New York
Rolfe DFS, Brown GC (1997) Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev 77:731–758
Saunders DK, Klemm DR (1994) Seasonal changes in the metabolic properties of muscle in blue-winged teal, Anas discors. Comp Biochem Physiol 107A:63–68
Selman C, Evans PR (2005) Alterations in tissue aerobic capacity may play a role in premigratory fattening in shorebirds. Biol Lett 1:101–104
Silvestri E, Schiavo L, Lombardi A, Goglia F (2005) Thyroid hormones as molecular determinants of thermogenesis. Acta Physiol Scand 184:265–283
Soboll S (1993) Thyroid hormone action on mitochondrial energy transfer. Biochim Biophys Acta 1144:1–16
Somjen D, Ismail-Beigi F, Edelman IE (1981) Nuclear binding of T3 and the effects on Qo2, Na–K-ATPase, and α-GPDH in liver and kidney. Am J Physiol 240:E146–E154
Stadie WC, Riggs BC (1944) Microtome for the preparation of tissue slices for metabolic studies of surviving tissues in vitro. J Biol Chem 154:697–690
Stearns S (1992) Evolution of life histories. Oxford University Press, Oxford
Vézina F, Williams TD (2003) Plasticity in body composition in breeding birds: what drives the metabolic cost of egg production? Physiol Biochem Zool 76:716–730
Vézina F, Williams TD (2005) Interaction between organ mass and citrate synthase activity as an indicator of tissue maximal oxidative capacity in breeding European Starlings: implications for metabolic rate and organ mass relationships. Funct Ecol 19:119–128
Weber TP, Piersma T (1996) Basal metabolic rate and the mass of tissues differing in metabolic scope: migration-related covariation between individual knots Calidris canutus. J Avian Biol 27:215–224
Acknowledgments
The study was supported by grants from the Norwegian Polar Institute and the Norwegian Science Research Council (no. 159584/V40) and the MariClim project (no. 165112/S30). The fieldwork by Olivier Chastel was financially and logistically supported by the Institut Paul-Émile Victor (IPEV Programme 330). Juli Broggi was financially supported by the Marie-Curie training site-ENDOCLIMA. Thanks are due to the staff at Kings Bay, the Sverdrup Station and the Rabot Station in Ny-Ålesund. We also thank Jorg Welcker for various help during the fieldwork, and André Lacroix for performing the T3 analyses at the CEBC. The study was approved by the Governor of Svalbard.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Heldmaier.
Rights and permissions
About this article
Cite this article
Rønning, B., Moe, B., Chastel, O. et al. Metabolic adjustments in breeding female kittiwakes (Rissa tridactyla) include changes in kidney metabolic intensity. J Comp Physiol B 178, 779–784 (2008). https://doi.org/10.1007/s00360-008-0268-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-008-0268-6