Abstract
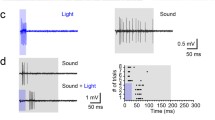
The barn owl, Tyto alba, extends its nictitating membrane (NM) in response to an air puff to the cornea or a mild para-orbital electrodermal shock. The NM extension habituated rapidly if the stimulus was repeated. Habituation was prevented by pairing the aversive stimulus with a sound. The sound stimulus did not, by itself, induce an NM extension. Repeated pairing of sound with the aversive stimulus caused the subjects to modify the duration of their NM extension, increasing the duration when exposed to longer aversive stimuli and decreasing in response to shorter stimuli. No transference of the response was seen from the aversive stimulus to the sound. The learned change in duration of the NM extension resisted extinction. This modification of the NM extension reflex resembles previous descriptions of primer-produced facilitation.









Similar content being viewed by others
Abbreviations
- NM:
-
Nictitating membrane
- NMR:
-
Nictitating membrane response
- SPL:
-
Sound pressure level
- dBA:
-
dB A-weighted sound pressure level
References
Bala AD, Takahashi TT (2000) Pupillary dilation response as an indicator of auditory discrimination in the barn owl. J Comp Physiol [A] 186(5):425–434
Bell CC (2001) Memory based expectations in sensory systems. Curr Opin Neurobiol 11(4):481–487
Coble SF, Robinson DE (1992) Discriminability of bursts of reproducible noise. J Acoust Soc Am 92(5):2630–2635
Darwin C (1872) The expression of the emotions in man and animals. The University of Chicago Press, Chicago
Davis JL, Coates SR (1978) Classical conditioning of the nictitating membrane response in the domestic chick. Physiol Psychol 6:7–10
Donegan NH (1981) Priming-produced facilitation or diminution of responding to a Pavlovian unconditioned stimulus. J Exp Psychol: Animal Behavior Processes 7:295–312
Donegan NH, Wagner AR (1987) Conditioned diminution and facilitation of the UR: a sometimes opponent-process interpretation. In: Goremezano I, Prokasy WF, Thompson RF (eds) Classical conditioning. Lawrence Erlbaum Associates, Hillsdale, NJ
Early SJ, Mason CR, Zheng L, Evilsizer M, Idrobo F, Harrison JM, Carney LH (2001) Studies of binaural detection in the rabbit (Oryctolagus cuniculus) with Pavlovian conditioning. Beh Neurosci 115:650–660
Grevert P, Moore JW (1970) The effect of unpaired US presentations on conditioning of the rabbit’s nictitating membrane responses. Psychonomic Sci 20:177–179
Hirsh IJ (1948) The influence of interaural phase on interaural summation and inhibition. J Acoust Soc Am 63:536–544
Hupka RB, Kwaterski SE, Moore JW (1970) Conditioned diminution of the UCR: differences between the human eyeblink and the rabbit nictitating membrane response. J Exp Psychol 83:45–51
Huxley TH (1866) Lessons in elementary physiology. Macmillan, London
Kidd G Jr, Mason CR, Rohtla TL, Deliwala PS (1998) Release from masking due to spatial separation of sources in the identification of nonspeech auditory patterns. J Acoust Soc Am 104(1):422–431
Kimble GA, Ost JWP (1961) A conditioned inhibitory process in eyelid conditioning. J Exp Psychol 36:150–156
Köppl C (1997) Frequency tuning and spontaneous activity in the auditory nerve and cochlear nucleus magnocellularis of the barn owl Tyto alba. J Neurophysiols 77:364–377
Licklider J (1948) The influence of interaural phase relations upon the masking of speech by white noise. J Acoust Soc Am 28:150–159
Manning KA, Evinger C (1986) Different forms of blinks and their two-stage control. Exp Brain Res 64:579–588
Oakley DA Russell IS (1974) Differential and reversal conditioning in partially neodecorticate rabbits. Physiol Behav 13:221–230
Pavlov IP (1927) Conditioned reflexes. Oxford University Press, London
Pennypacker HS, King FA, Achenbach KE, Roberts L (1966) An apparatus and procedure for conditioning the eye-blink reflex in the squirrel monkey. J Exp Anal Behavior 9:601–604
Pinsker H, Kupfermann I, Castellucci V, Kandel E (1970) Habituation and dishabituation of the gill-withdrawal reflex in Aplysia. Science 167:1740–1742
Plotkin HC, Oakley DA (1975) Backward conditioning in the rabbit (Oryctolagus cuniculus). J Comp Physiol Psychol 88:586–590
Schneiderman N, Fuentes I, Gormezano I (1962) Acquisition and extinction of the classically conditioned eyelid response in the albino rabbit. Science 136:650–652
Sokolov EN (1958) The orienting reflex, its structure and mechanisms. In: Voronin LG, Leontiev AN, Luria AR, Sokolov EN, Vinogradova OS (eds) Orienting reflex and exploratory behavior. Publishing House of The Academy of Pedagogical Sciences of RSFR, Moscow, pp 141–153
Solomon PR, Moore JW (1975) Latent inhibition and stimulus generalization of the classically conditioned nictitating membrane response in rabbits (Oryctolagus cuniculus) following dorsal hippocampal ablation. J Comp Physiol Psychol 89:1192–1203
Spence KW (1966) Cognitive and drive factors in the extinction of the conditioned eye blink in human subjects. Psychol Rev 73:445–458
Svensson P, Ivarsson M, Hesslow G (1997) Effect of varying the intensity and train frequency of forelimb and cerebellar mossy fiber conditioned stimuli on the latency of conditioned eye-blink responses in decerebrate ferrets. Learning & Memory 4:105–115
Takahashi TT, Keller CH (1994) Representation of multiple sound sources in the owl’s auditory space map. J Neurosci 14:4780–4793
Thompson RF, Spencer WA (1966) Habituation: a model phenomenon for the study of neuronal substrates of behavior. Psychol Rev 73:16–43
Wagner AR (1981) SOP: A model of automatic memory processing in animal behavior. In: Spear NE, Miller RR (eds) Information processing in animals: Memory mechanisms. Lawrence Erlbaum Associates, Hillsdale, NJ
Weisz DJ, LoTurco JJ (1988) Reflex facilitation of the nictitating membrane response remains after cerebellar lesions. Behav Neurosci 102:203–209
Wild JM (1999) Trigeminal disynaptic circuit mediating corneal afferent input to M. depressor palpebrae inferioris motoneurons in the pigeon (Columba livia). J Comp Neurol 403:391–406
Woody CD, Brozek G (1969) Conditioned eye blink in the cat: evoked responses of short latency. Brain Res 12:257–260
Acknowledgements
This work was supported by grants from the National Institute of Deafness and Communication Disorders (DC03925), Medical Research Foundation of Oregon, and the McKnight Foundation for Neuroscience. Dr Matthew W. Spitzer, Dr Clifford H. Keller, Dr Michael L. Spezio, Dr Michael Anderson, Dr Richard T. Marrocco, Dr Marvin Gordon-Lickey and Dr Norman Weinberger provided us with many helpful suggestions and insights. Dr. Matt Spitzer kindly shared his photograph of the owl used in Fig. 1a. Dr Keller provided invaluable technical assistance with hardware and software.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bala, A.D.S., Takahashi, T.T. Learned modification of the nictitating membrane reflex by auditory stimuli in the barn owl. J Comp Physiol A 191, 627–637 (2005). https://doi.org/10.1007/s00359-005-0614-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00359-005-0614-z