Abstract
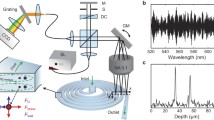
In vivo acquisition of endothelial wall shear stress requires instantaneous depth-resolved whole-field pulsatile flow profile measurements in microcirculation. High-accuracy, quantitative and non-invasive velocimetry techniques are essential for emerging real-time mechano-genomic investigations. To address these research needs, a novel biological flow quantification technique, OCT-μPIV, was developed utilizing high-speed optical coherence tomography (OCT) integrated with microscopic Particle Image Velocimetry (μPIV). This technique offers the unique advantage of simultaneously acquiring blood flow profiles and vessel anatomy along arbitrarily oriented sagittal planes. The process is instantaneous and enables real-time 3D flow reconstruction without the need for computationally intensive image processing compared to state-of-the-art velocimetry techniques. To evaluate the line-scanning direction and speed, four sets of parametric synthetic OCT-μPIV data were generated using an in-house code. Based on this investigation, an in vitro experiment was designed at the fastest scan speed while preserving the region of interest providing the depth-resolved velocity profiles spanning across the width of a micro-fabricated channel. High-agreement with the analytical flow profiles was achieved for different flow rates and seed particle types and sizes. Finally, by employing blood cells as non-invasive seeding particles, in vivo embryonic vascular velocity profiles in multiple vessels were measured in the early chick embryo. The pulsatile flow frequency and peak velocity measurements were also acquired with OCT-μPIV, which agreed well with previous reported values. These results demonstrate the potential utility of this technique to conduct practical microfluidic and non-invasive in vivo studies for embryonic blood flows.





Similar content being viewed by others
References
Bogren HG, Buonocore MH, Valente RJ (2004) Four-dimensional magnetic resonance velocity mapping of blood flow patterns in the aorta in patients with atherosclerotic coronary artery disease compared to age-matched normal subjects. J Magn Reson Imaging 19(4):417–427
Boulnois J-L (1986) Photophysical processes in recent medical laser developments: a review. Lasers Med Sci 1:47–66
Brezinski ME (2006) Optical coherence tomography: principles and applications. Academic Press, London
Chen J, Katz J (2005) Elimination of peak-locking error in PIV analysis using the correlation mapping method. Meas Sci Technol 16(8):1605–1618
Chen C-Y, Patrick MJ, Corti P, Kowalski W, Roman BL, Pekkan K (2011) Analysis of early embryonic great-vessel microcirculation in zebrafish using high-speed confocal μPIV. Biorheology 48:305–321
Corti P, Young S, Chen CY, Patrick MJ, Rochon ER, Pekkan K, Roman BL (2011) Interaction between alk1 and blood flow in the development of arteriovenous malformations. Development 138(8):1573–1582
Davis A, Izatt J, Rothenberg F (2009) Quantitative measurement of blood flow dynamics in embryonic vasculature using spectral Doppler velocimetry. Anat Record (Hoboken) 292(3):311–319
Drexler W, Fujimoto JG (2008) Optical coherence tomography: technology and applications (biological and medical physics, biomedical engineering). Springer, Berlin
Fercher AF, Drexler W, Hitzenberger CK, Lasser T (2003) Optical coherence tomography—principles and applications. Rep Prog Phys 66:239–303
Frakes DH, Pekkan K, Dasi LP, Kitajima HD, de Zelicourt D, Leo HL, Carberry J, Sundareswaran K, Simon H, Yoganathan AP (2008) Modified control grid interpolation for the volumetric reconstruction of fluid flows. Exp Fluids 45(6):987–997
Hahn C, Schwartz MA (2009) Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol 10(1):53–62
Hove JR, Koster RW, Forouhar AS, Acevedo-Bolton G, Fraser SE, Gharib M (2003) Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature 421(6919):172–177
Hu N, Clark EB (1989) Hemodynamics of the stage 12 to stage 29 chick embryo. Circ Res 65(6):1665–1670
Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, Hee MR, Flotte T, Gregory K, Puliafito CA et al (1991) Optical coherence tomography. Science 254(5035):1178–1181
Kim GB, Lee SJ (2006) X-ray PIV measurements of blood flows without tracer particles. Exp Fluids 41(2):195–200
Kim HB, Hertzberg JR, Shandas R (2004) Development and validation of echo PIV. Exp Fluids 36(3):455–462
Kowalski WJ, Teslovich NC, Dur O, Keller BB, Pekkan K (2012) Computational hemodynamic optimization predicts dominant aortic arch selection is driven by embryonic outflow tract orientation in the chick embryo. Biomech Model Mechanobiol 11(7):1057–1073
Lara M, Chen CY, Mannor P, Dur O, Menon PG, Yoganathan AP, Pekkan K (2011) Hemodynamics of the hepatic venous three-vessel confluences using particle image velocimetry. Ann Biomed Eng 39(9):2398–2416
Lee JY, Ji HS, Lee SJ (2007) Micro-PIV measurements of blood flow in extraembryonic blood vessels of chicken embryos. Physiol Meas 28(10):1149–1162
Lima R, Wada S, Tsubota K, Yamaguchi T (2006) Confocal micro-PIV measurements of three-dimensional profiles of cell suspension flow in a square microchannel. Meas Sci Technol 17(4):797–808
Lindken R, Westerweel J, Wieneke B (2006) Stereoscopic micro particle image velocimetry. Exp Fluids 41(2):161–171
Lindken R, Rossi M, Grosse S, Westerweel J (2009) Micro-particle image velocimetry (microPIV): recent developments, applications, and guidelines. Lab Chip 9(17):2551–2567
Markl M, Harloff A, Bley TA, Zaitsev M, Jung B, Weigang E, Langer M, Hennig J, Frydrychowicz A (2007) Time-resolved 3D MR velocity mapping at 3T: improved navigator-gated assessment of vascular anatomy and blood flow. J Magn Reson Imaging 25(4):824–831
Oosterbaan AM, Ursem NT, Struijk PC, Bosch JG, van der Steen AF, Steegers EA (2009) Doppler flow velocity waveforms in the embryonic chicken heart at developmental stages corresponding to 5–8 weeks of human gestation. Ultrasound Obstet Gynecol 33(6):638–644
Park JS, Kihm KD (2006) Three-dimensional micro-PTV using deconvolution microscopy. Exp Fluids 40(3):491–499
Park JS, Choi CK, Kihm KD (2004) Optically sliced micro-PIV using confocal laser scanning microscopy (CLSM). Exp Fluids 37(1):105–119
Parrish JA (1981) New concepts in therapeutic photomedicine: photochemistry, optical targeting and the therapeutic window. J Investig Dermatol 1:45–50
Patrick MJ, Chen CY, Frakes D, Dur O, Pekkan K (2011) Cellular-level near-wall unsteadiness of high-hematocrit erythrocyte flow using confocal μPIV. Exp Fluids 50:887–904
Poelma C, Vennemann LindkenR, Westerweel J (2008) In vivo blood flow and wall shear stress measurements in the vitelline network. Exp Fluids 45(4):703–713
Roman BL, Pekkan K (2012) Mechanotransduction in embryonic vascular development. Biomech Model Mechanobiol
Srinivasan S, Baldwin HS, Aristizabal O, Kwee L, Labow M, Artman M, Turnbull DH (1998) Noninvasive, in utero imaging of mouse embryonic heart development with 40-MHz echocardiography. Circulation 98(9):912–918
Sundareswaran KS, Haggerty CM, de Zelicourt D, Dasi LP, Pekkan K, Frakes DH, Powell AJ, Kanter KR, Fogel MA, Yoganathan AP (2011) Visualization of flow structures in Fontan patients using 3-dimensional phase contrast magnetic resonance imaging. J Thorac Cardiovasc Surg 143(5):1108–1116
Wang Y, Dur O, Patrick MJ, Tinney JP, Tobita K, Keller BB, Pekkan K (2009) Aortic arch morphogenesis and flow modeling in the chick embryo. Ann Biomed Eng 37(6):1069–1081
Weinbaum S, Tarbell JM, Damiano ER (2007) The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng 9:121–167
Weissmann-Brenner A, Pretorius DH, Achiron R, Gindes L (2012) Fetal echocardiography: the four-chamber view, the outflow tracts, and the contribution of the cardiac arches. Ultrasound Clin 7(1):1–13
Zheng H, Liu L, Williams L, Hertzberg R, Lanning C, Shandas R (2006) Real time multicomponent echo particle image velocimetry technique for opaque flow imaging. Appl Phys Lett 88(26):261915
Acknowledgments
This work was supported by NSF CAREER 0954465 and ERC Consolidator Grants.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
In PIV, a significant portion of measurement error can be caused by the tracer particle size. This error have been well established for a standard μPIV experiment (Lindken et al. 2009) but needs to be quantified for the new OCT-μPIV technique. In order to investigate particle size effect on the OCT-μPIV velocity measurements, three different types of particles were tested that range from 1 to 12 μm. The resultant velocity profiles obtained with these particles were plotted in Fig. 6 along with corresponding analytical solution comparison and the raw OCT-μPIV images. Through this comparison, the optimal ratio of particle size to the relevant fluid dynamic length scale should be in the range of 0.03 (12/400 μm) and 0.003 (1/400 μm). In this work, the channel width was fabricated to be 400 μm, and therefore, the particle size of 3.2 μm was selected to ensure that the particle response time is smaller than the smallest time scale in the flow and free of Brownian motion effects. The calculated interparticular distance values for 12, 3.2, and 1.0 μm particles are 94, 38, and 16.7 μm, respectively.
Depth-resolved velocity profiles acquired at the center of the microchannel with three different types of particles compared with the corresponding analytical solutions. Error bars indicate one standard deviation values for each particle type. Red: hollow glass beads with 8–12 μm in diameter (TSI, Inc., MN) at concentration of 0.6 % (mg/mL); Blue: fluorescent particles with 3.2 μm in diameter (Microgenics, Inc., CA) at concentration of 0.2 %; Green: fluorescent particles with 1.0 μm in diameter (Microgenics, Inc., CA) at concentration of 0.1 %
Rights and permissions
About this article
Cite this article
Chen, CY., Menon, P.G., Kowalski, W. et al. Time-resolved OCT-μPIV: a new microscopic PIV technique for noninvasive depth-resolved pulsatile flow profile acquisition. Exp Fluids 54, 1426 (2013). https://doi.org/10.1007/s00348-012-1426-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00348-012-1426-x