Abstract
Purpose
Combined androgen blockade (CAB) and luteinizing hormone-releasing hormone (LHRH) agonist monotherapy are commonly used in androgen deprivation therapy (ADT). In this randomized clinical trial, we aimed to compare the two methods of ADT in terms of quality of life (QOL).
Methods
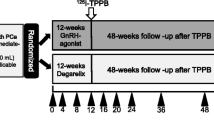
Eighty patients who underwent primary ADT for newly diagnosed prostate cancer were randomly assigned to CAB group (Group 1) and LHRH agonist monotherapy group (Group 2). Leuprolide and anti-androgen (bicalutamide 50 mg) were used to minimize the confounding effects caused by medication. QOL was evaluated at baseline, 3 months and 6 months post-ADT using validated EORTC QLQ-C30, PR25, and depression questionnaires. A difference of > 10 points in the EORTC domain scores was defined as ‘clinically significant’.
Results
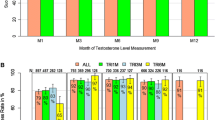
In the baseline characteristics, there was no significant difference between the two groups. At 3 months after ADT, Group 1 had significantly lower pain scores than Group 2 (p = 0.004), while Group 1 had significantly poorer diarrhea symptom score than Group 2, without clinical significance (p = 0.047). No significant differences were observed in the C30, PR25 domains, and the depression score at 3 months. At 6 months, the QOL scores of all the groups were similar.
Conclusions
There was no difference in the patient’s QOL, except that CAB group was associated with significantly better pain relief than LHRH agonist monotherapy at 3 months following ADT, which was not sustained thereafter. Our results suggest that the benefit of prolonged (≥ 3 months) CAB is questionable in terms of patients’ QOL.
Similar content being viewed by others
References
Sharifi N, Gulley JL, Dahut WL (2005) Androgen deprivation therapy for prostate cancer. JAMA 294(2):238–244. https://doi.org/10.1001/jama.294.2.238
Isbarn H, Boccon-Gibod L, Carroll PR, Montorsi F, Schulman C, Smith MR, Sternberg CN, Studer UE (2009) Androgen deprivation therapy for the treatment of prostate cancer: consider both benefits and risks. Eur Urol 55(1):62–75. https://doi.org/10.1016/j.eururo.2008.10.008
Prostate Cancer Trialists’ Collaborative Group (2000) Maximum androgen blockade in advanced prostate cancer: an overview of the randomised trials. Lancet 355(9214):1491–1498
Samson DJ, Seidenfeld J, Schmitt B, Hasselblad V, Albertsen PC, Bennett CL, Wilt TJ, Aronson N (2002) Systematic review and meta-analysis of monotherapy compared with combined androgen blockade for patients with advanced prostate carcinoma. Cancer 95(2):361–376. https://doi.org/10.1002/cncr.10647
Akaza H (2011) Combined androgen blockade for prostate cancer: review of efficacy, safety and cost-effectiveness. Cancer Sci 102(1):51–56. https://doi.org/10.1111/j.1349-7006.2010.01774.x
Akaza H, Hinotsu S, Usami M, Arai Y, Kanetake H, Naito S, Hirao Y, Study Group for the Combined Androgen Blockade Therapy of Prostate C (2009) Combined androgen blockade with bicalutamide for advanced prostate cancer: long-term follow-up of a phase 3, double-blind, randomized study for survival. Cancer 115(15):3437–3445. https://doi.org/10.1002/cncr.24395
Crawford ED, Eisenberger MA, McLeod DG, Spaulding JT, Benson R, Dorr FA, Blumenstein BA, Davis MA, Goodman PJ (1989) A controlled trial of leuprolide with and without flutamide in prostatic carcinoma. N Engl J Med 321(7):419–424. https://doi.org/10.1056/NEJM198908173210702
Eisenberger MA, Blumenstein BA, Crawford ED, Miller G, McLeod DG, Loehrer PJ, Wilding G, Sears K, Culkin DJ, Thompson IM Jr, Bueschen AJ, Lowe BA (1998) Bilateral orchiectomy with or without flutamide for metastatic prostate cancer. N Engl J Med 339(15):1036–1042. https://doi.org/10.1056/NEJM199810083391504
Shahinian VB, Kuo YF, Freeman JL, Goodwin JS (2006) Determinants of androgen deprivation therapy use for prostate cancer: role of the urologist. J Natl Cancer Inst 98(12):839–845. https://doi.org/10.1093/jnci/djj230
Krahn M, Bremner KE, Tomlinson G, Luo J, Ritvo P, Naglie G, Alibhai SM (2011) Androgen deprivation therapy in prostate cancer: are rising concerns leading to falling use? BJU Int 108(10):1588–1596. https://doi.org/10.1111/j.1464-410X.2011.10127.x
Park J, Suh B, Shin DW, Hong JH, Ahn H (2016) Changing patterns of primary treatment in Korean men with prostate cancer over 10 years: a nationwide population based study. Cancer Res Treat 48(3):899–906. https://doi.org/10.4143/crt.2015.212
Moinpour CM, Savage MJ, Troxel A, Lovato LC, Eisenberger M, Veith RW, Higgins B, Skeel R, Yee M, Blumenstein BA, Crawford ED, Meyskens FL Jr (1998) Quality of life in advanced prostate cancer: results of a randomized therapeutic trial. J Natl Cancer Inst 90(20):1537–1544
Arai Y, Akaza H, Deguchi T, Fujisawa M, Hayashi M, Hirao Y, Kanetake H, Naito S, Namiki M, Tachibana M, Usami M, Ohashi Y (2008) Evaluation of quality of life in patients with previously untreated advanced prostate cancer receiving maximum androgen blockade therapy or LHRHa monotherapy: a multicenter, randomized, double-blind, comparative study. J Cancer Res Clin Oncol 134(12):1385–1396. https://doi.org/10.1007/s00432-008-0409-z
Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman SB, de Haes JC et al (1993) The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85(5):365–376
Yun YH, Park YS, Lee ES, Bang SM, Heo DS, Park SY, You CH, West K (2004) Validation of the Korean version of the EORTC QLQ-C30. Qual Life Res 13(4):863–868. https://doi.org/10.1023/B:QURE.0000021692.81214.70
van Andel G, Bottomley A, Fossa SD, Efficace F, Coens C, Guerif S, Kynaston H, Gontero P, Thalmann G, Akdas A, D’Haese S, Aaronson NK (2008) An international field study of the EORTC QLQ-PR25: a questionnaire for assessing the health-related quality of life of patients with prostate cancer. Eur J Cancer 44(16):2418–2424. https://doi.org/10.1016/j.ejca.2008.07.030
Park J, Shin DW, Yun SJ, Park SW, Jeon SS, Kwak C, Kwon TG, Kim HJ, Ahn H (2013) Cross-cultural application of the Korean version of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire for patients with prostate cancer—EORTC QLQ-PR25. Oncology 85(5):299–305. https://doi.org/10.1159/000355689
Han C, Jo SA, Kwak JH, Pae CU, Steffens D, Jo I, Park MH (2008) Validation of the Patient Health Questionnaire-9 Korean version in the elderly population: the Ansan Geriatric study. Compr Psychiatry 49(2):218–223. https://doi.org/10.1016/j.comppsych.2007.08.006
Maringwa J, Quinten C, King M, Ringash J, Osoba D, Coens C, Martinelli F, Reeve BB, Gotay C, Greimel E, Flechtner H, Cleeland CS, Schmucker-Von Koch J, Weis J, Van Den Bent MJ, Stupp R, Taphoorn MJ, Bottomley A, Project EP, Brain Cancer G (2011) Minimal clinically meaningful differences for the EORTC QLQ-C30 and EORTC QLQ-BN20 scales in brain cancer patients. Ann Oncol 22(9):2107–2112. https://doi.org/10.1093/annonc/mdq726
Cornford P, Bellmunt J, Bolla M, Briers E, De Santis M, Gross T, Henry AM, Joniau S, Lam TB, Mason MD, van der Poel HG, van der Kwast TH, Rouviere O, Wiegel T, Mottet N (2017) EAU-ESTRO-SIOG guidelines on prostate cancer. Part II: treatment of relapsing, metastatic, and castration-resistant prostate cancer. Eur Urol 71(4):630–642. https://doi.org/10.1016/j.eururo.2016.08.002
Sanda MG, Cadeddu JA, Kirkby E, Chen RC, Crispino T, Fontanarosa J, Freedland SJ, Greene K, Klotz LH, Makarov DV, Nelson JB, Rodrigues G, Sandler HM, Taplin ME, Treadwell JR (2018) Clinically localized prostate cancer: AUA/ASTRO/SUO Guideline. Part II: recommended approaches and details of specific care options. J Urol 199(4):990–997. https://doi.org/10.1016/j.juro.2018.01.002
da Silva FC, Reis E, Costa T, Denis L (1993) Quality of life in patients with prostatic cancer. A feasibility study. The Members of Quality of Life Committee of the EORTC Genitourinary Group. Cancer 71(3 Suppl):1138–1142
Gagliano-Juca T, Travison TG, Nguyen PL, Kantoff PW, Taplin ME, Kibel AS, Manley R, Hally K, Bearup R, Beleva YM, Huang G, Edwards RR, Basaria S (2018) Effects of androgen deprivation therapy on pain perception, quality of life, and depression in men with prostate cancer. J Pain Symptom Manag 55(2):307–317 e301. https://doi.org/10.1016/j.jpainsymman.2017.09.017
Kumar RJ, Barqawi A, Crawford ED (2005) Adverse events associated with hormonal therapy for prostate cancer. Rev Urol 7(Suppl 5):S37–S43
Langenstroer P, Porter HJ 2nd, McLeod DG, Thrasher JB (2004) Direct gastrointestinal toxicity of flutamide: comparison of irradiated and nonirradiated cases. J Urol 171(2 Pt 1):684–686. https://doi.org/10.1097/01.ju.0000106835.60202.81
Washino S, Hirai M, Saito K, Kobayashi Y, Arai Y, Miyagawa T (2018) Impact of androgen deprivation therapy on volume reduction and lower urinary tract symptoms in patients with prostate cancer. Low Urin Tract Symptoms 10(1):57–63. https://doi.org/10.1111/luts.12142
Axcrona K, Aaltomaa S, da Silva CM, Ozen H, Damber JE, Tanko LB, Colli E, Klarskov P (2012) Androgen deprivation therapy for volume reduction, lower urinary tract symptom relief and quality of life improvement in patients with prostate cancer: degarelix vs goserelin plus bicalutamide. BJU Int 110(11):1721–1728. https://doi.org/10.1111/j.1464-410X.2012.11107.x
Dinh KT, Reznor G, Muralidhar V, Mahal BA, Nezolosky MD, Choueiri TK, Hoffman KE, Hu JC, Sweeney CJ, Trinh QD, Nguyen PL (2016) Association of androgen deprivation therapy with depression in localized prostate cancer. J Clin Oncol 34(16):1905–1912. https://doi.org/10.1200/JCO.2015.64.1969
Wadman M (2015) Treatment: when less is more. Nature 528:S126. https://doi.org/10.1038/528S126a
Herr HW, O’Sullivan M (2000) Quality of life of asymptomatic men with nonmetastatic prostate cancer on androgen deprivation therapy. J Urol 163(6):1743–1746
Acknowledgements
This study was supported by DAEWOONG Pharm, Co. Research Grant.
Author information
Authors and Affiliations
Contributions
HSP data analysis, manuscript writing/editing. HBS data analysis, manuscript writing/editing. SHW data collection. SHJ data collection. SHL data collection. SHK data collection. JSS data collection. DWS data collection, data analysis. JP project development, data collection, data analysis, manuscript writing/editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the Institutional Review Board of the Eulji University Hospital (No. 2014-03-011).
Informed consent
A written informed consent was obtained from each patient before screening.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 2 (TIFF 12032 kb) Supplementary Figure S2. Patient disposition and study flow
345_2019_2847_MOESM3_ESM.tif
Supplementary material 3 (TIFF 2743 kb) Supplementary Figure S3. Changes in hormonal treatment-related symptom according to ADT method
Rights and permissions
About this article
Cite this article
Park, H.S., Shin, H.B., Woo, S.H. et al. Combined androgen blockade (CAB) versus luteinizing hormone-releasing hormone (LHRH) agonist monotherapy for androgen deprivation therapy. World J Urol 38, 971–979 (2020). https://doi.org/10.1007/s00345-019-02847-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-019-02847-y