Abstract
Objective
To compare the radiological and clinical outcomes of endoscopic treatment of primary VUR using polyacrylate-polyalcohol copolymer (PPC-Vantris®) or dextranomer-hyaluronic acid copolymer (Dx/HA-Deflux®).
Materials and methods
From October 2014 to April 2017, patients with primary VUR grade III to V that needed endoscopic treatment (ET) were eligible for this randomized clinical trial. We excluded toilet-trained patients with lower urinary tract symptoms. Patients were randomized and allocated into two groups: PPC group and Dx/HA group. After endoscopic treatment a voiding cystourethrography (VCUG) was performed at 6 months; if VUR was still present a second ET was performed. Radiological success was considered if postoperative VUR grade was 0 and clinical success rate was considered if no more fUTI appeared during follow-up.
Results
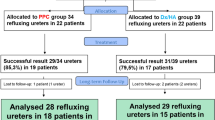
Forty-six patients were eligible but 2 did not accept the trial. Forty-four patients with 73 refluxing ureters were included. PPC: 34 refluxing ureters; and Dx/HA: 39 refluxing ureters. Both groups were statistically homogeneous and comparable. Mean follow-up was 27.6 months. Radiological success rate (82.2%) and clinical success rate (92.3%) were similar in both groups (p > 0.05). The volume of bulking agent used in those successfully treated was greater in Dx/HA group (p < 0.05). Distal ureter was excise in all cases of ureteral reimplantation after PPC treatment; however, distal ureter was preserved in all ureters reimplanted after Dx/HA injection.
Conclusion
PPC and Dx/HA had similar outcomes, but we must warn that ureteral reimplantation after endoscopic treatment with PPC is difficult because of the periureteral fibrosis.

Similar content being viewed by others
References
Kirsch AJ, Perez-Brayfield M, Smith EA, Scherz H (2004) The modified STING procedure to correct vesicoureteral reflux: improved results with submucosal implantation within the intramural ureter. J Urol 171:2413
Läckgren G, Wählin N (2001) Sk€oldenberg E, Stenberg A. Long-term followup of children treated with dextranomer/hyaluronic acid copolymer for vesicoureteral reflux. J Urol 166:1887–1892
García-Aparicio L, Blázquez-Gómez E, Vila Santandreu A, Camacho JA, Vila-Cots J, Ramos M et al (2016) Routine delayed voiding cystourethography after successful endoscopic treatment with Dx/HA of vesicoureteral reflux. Is it necessary? Actas Urol Esp 40(10):635–639
Lee EK, Gatti JM, Demarco RT et al (2009) Long-term follow-up of dextranomer/hyaluronic acid injection for vesicoureteral reflux: late failure warrants continued follow-up. J Urol 181:1869–1874
Ormaechea M, Ruiz E, Denes E et al (2010) New tissue bulking agent (polyacrylate polyalcohol) for treating vesicoureteral reflux: preliminary results in children. J Urol 183:714–717
Chertin B, Arafeh WA, Zeldin A et al (2011) Preliminary data on endoscopic treatment of vesicoureteric reflux with polyacrylate polyalcohol copolymer (Vantris): surgical outcome following single injection. J Pediatr Urol 7:654–657
Sharifiaghdas F, Tajalli F, Otukesh H, Shamsabadi RH (2014) Endoscopic correction of primary VUR by using polyacrylate polyalcohol copolymer (Vantris) in young girls: 2-year follow-up. J Pediatr Urol 10:1032–1369
Chertin B, Arafeh WA, Zeldin A, Ostrovsky IA, Kocherov S (2013) Endoscopic correction of VUR using Vantris as a new non-biodegradable tissue augmenting substance: three years of prospective follow-up. Urology 82:201–204
Warchol S, Krzemien G, Szmigielska A, Bombinski P, Brzewski M, Dudek-Warchol T (2016) Comparison of results of endoscopic correction of vesicoureteral reflux in children using two bulking substances: dextranomer/hyaluronic acid copolymer (Deflux) versus polyacrylate-polyalcohol copolymer (Vantris). J Pediatr Urol 12(4):256.e1–256.e4
Taşkinlar H, Avlan D, Bahadir GB, Delibaş A, Nayci A (2016) The outcomes of two different bulking agents (dextranomer hyaluronic acid copolymer and polyacrylate-polyalcohol copolymer) in the treatment of primary vesico-ureteral reflux. Int Braz J Urol 42(3):514–520
Kocaoglu C (2016) Endoscopic treatment of grades IV and V vesicoureteral reflux with two bulking substances: dextranomer hyaluronic acid copolymer versus polyacrylate polyalcohol copolymer in children. J Pediatr Surg 51(10):1711–1715
Karakus SC, User İR, Kılıc BD, Akçaer V, Ceylan H, Ozokutan BH (2016) The comparison of dextranomer/hyaluronic acid and polyacrylate-polyalcohol copolymers in endoscopic treatment of vesicoureteral reflux. J Pediatr Surg 51(9):1496–1500
Matouschek E (1981) Treatment of vesicorenal reflux by transurethral teflon-injection (author’s transl). Urol A 20(5):263–264
García-Aparicio L, Rodo J, Palazon P, Martín O, Blázquez-Gómez E, Manzanares A et al (2013) Acute and delayed vesicoureteral obstruction after endoscopic treatment of primary vesicoureteral reflux with dextranomer/hyaluronic acid copolymer: why and how to manage. J Pediatr Urol 9(4):493–497
Alizadeh F, Mazdak H, Khorrami MH, Khalighinejad P, Shoureshi P (2013) Postoperative ureteral obstruction after endoscopic treatment of vesicoureteral reflux with polyacrylate polyalcohol copolymer (Vantris®). J Pediatr Urol 9(4):488–492
Kocherov S, Ulman I, Nikolaev S, Corbetta JP, Rudin Y, Slavkovic A et al (2014) Multicenter survey of endoscopic treatment of vesicoureteral reflux using polyacrylate-polyalcohol bulking copolymer (Vantris). Urology 84(3):689–693
Funding
No financial support.
Author information
Authors and Affiliations
Contributions
LG-A: protocol/project development, data collection or management, data analysis, manuscript writing/editing. EB-G: protocol/project development, data analysis, manuscript writing/editing. OM-S: protocol/project development, data collection or management, data analysis. SP-B: protocol/project development, data collection or management. JA: data collection or management. AS: data collection or management. XT: protocol/project development.
Corresponding author
Ethics declarations
Conflict of interest
We have no conflict of interest.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by our Research Ethical Board number: 45-14.
Informed consent
All patients included in the study signed the informed consent.
Rights and permissions
About this article
Cite this article
García-Aparicio, L., Blázquez-Gómez, E., Martin, O. et al. Randomized clinical trial between polyacrylate-polyalcohol copolymer (PPC) and dextranomer-hyaluronic acid copolymer (Dx/HA) as bulking agents for endoscopic treatment of primary vesicoureteral reflux (VUR). World J Urol 36, 1651–1656 (2018). https://doi.org/10.1007/s00345-018-2314-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-018-2314-7