Abstract
Objectives
Previous studies suggest that maximum tumor diameter (MTD) is a predictor of recurrence in prostate cancer (PC). This study investigates the prognostic value of MTD for biochemical recurrence (BCR) in patients with PC, after radical prostatectomy (RP), with emphasis on high-risk localized prostate cancer.
Methods
RP specimens of 542 patients were evaluated with a median follow-up of 39.5 months (range 0.6–150 months). MTD was defined as the largest diameter of the largest tumor; high-risk as ≥T2c or PSA level > 20 ng/ml or Gleason score ≥8 and BCR as two consecutive PSA levels > 0.10 ng/ml. Proportional hazards multivariable regression models were composed to determine prognostic factors for BCR.
Results
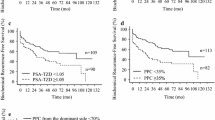
Overall, 114 patients developed BCR after RP. The overall 5-year risk of BCR was 25% (95% CI = 20.4–29.6), and median MTD was 24 mm (range 1–65). MTD in the total and high-risk group was associated with total tumor volume, volume of the largest tumor, pre-operative PSA levels, and Gleason score. In a univariable analyses, MTD was weakly associated with risk of BCR (HR = 1.02 per mm increase, 95% CI = 1.002–1.035, P = 0.024) in the total group; in the high-risk group this association was lost (HR = 1.01, 95%CI = 0.99–1.03, P = 0.18). Multivariable analyses indicated that positive surgical margins, higher Gleason score, advanced pathological stage, and multiple tumors were the main prognostic factors for BCR irrespective of the risk profile. MTD did not provide additional information.
Conclusions
MTD is not an independent prognostic factor for BCR in patients treated with RP, irrespective of the risk profile.
Similar content being viewed by others
Introduction
The characteristics mostly used to predict progression of prostate cancer (PC), after radical prostatectomy (RP), are Gleason score, pre-operative PSA level, pathological staging, positive surgical margins, and tumor volume (TV). [1] The first three are also used to define high-risk localized PC [2]. TV in the RP specimen is also clearly associated with prognosis. However, whether it is an independent prognostic factor, both for the total group and for the high-risk group, still remains controversial [3].
Epstein et al. [4] stated that, although TV is a predictor of progression, it did not provide additional information over Gleason score and pathological stage. This was also found in a study by Salomon et al. [5] where only Gleason score and pathological stage were independent factors to predict progression after RP. If these parameters were known, TV did not provide any additional information. This is in contrast with Stamey et al. [6] who found that TV is an independent prognostic parameter for PC progression. These findings were also observed in other studies [7–9]. None of these studies, however, specifically addressed the high-risk subgroup. An explanation of these conflicting results might be that TV estimations can be done with the help of several techniques, as Humphrey and Volmer [10] described earlier. Moreover, these techniques are rather complex and time-consuming. Therefore, maximum tumor diameter (MTD) has been suggested as a fast and easy proxy for TV. Renshaw et al. [11] noted that simple measurements of the largest diameter of the largest tumor from glass slides is a predictor of PSA failure. This was supported by Eichelberger et al. [12] who showed that MTD correlates with TV and other potential prognostic factors for clinical outcome. However, these studies had either a small number of patients with a relatively short follow-up (57 patients, median follow-up 27.2 months) or did not investigate the correlation between MTD and biochemical recurrence (BCR).
Therefore, in this present study, with a large cohort of 542 RP specimens, and a median follow-up of 39.5 months, we studied the prognostic value of MTD, as a surrogate for tumor volume, for BCR in patients with PC, with special attention to the high-risk group.
Materials and methods
Between 1992 and 2005, 617 patients were treated with RP for clinically localized PC at our institute. Of these patients, 75 were excluded from analyses because of HIFU treatment, hormonal pre-treatment or irradiation before RP. Of the remaining 542 patients, five had incomplete follow-up data. Charts were examined retrospectively for clinical follow-up data and risk group classification. These risk groups were classified according to D’Amico et al. [2]. High risk was defined as stage ≥T2c or PSA level >20 ng/ml or Gleason score ≥8, and we formed one group of the low and intermediate risk group (stage < T2c, PSA level ≤ 20 ng/ml and Gleason score ≤ 7).
All RP specimens were fixed overnight, inked, and cut into serial, transverse, 4 mm thick slices according to a standard protocol by Ruijter et al. [13]. In brief, the apical and basal margins were amputated to a thickness of 4 mm and sectioned parasagittally at 4 mm intervals. The seminal vesicles were sectioned parallel and entirely submitted. The prostate was then sectioned perpendicularly to the long axis (apical to basal) of the gland along the posterior rectal surface at 4-mm intervals. Tumor was outlined on the microscopic glass slides and schematically drawn on the macroscopically photographed transverse sections in order to be able to reconstruct tumor extension and tumor multifocallity. Detailed pathological analysis, including number of tumors, total volume of all tumors, MTD, pathological staging, Gleason score, extracapsular extension and positive margins, was performed by a single pathologist (CAHK). Tumors were staged using the American Joint Committee on Cancer (AJCC) TNM staging criteria of 2002 [14]. MTD was defined as the largest diameter of the largest tumor. MTD was determined by marking both ends of the tumor and measuring the distance at the glass slide directly. If the tumor was present on consecutive glass slides, thickness of the slices of the concerning sections was summed. This reconstructed diameter was considered as MTD if it was larger than the diameter, and was visualized directly on the glass slides.
PSA values were obtained before surgery and at every follow-up point. BCR after RP was defined as two subsequent PSA levels above 0.10 ng/ml among patients who reached non-measurable levels after RP.
Statistical analysis
Baseline characteristics are summarized with median and interquartile ranges (IQR). Associations between MTD and clinical or pathological characteristics were examined by univariate regression models. Kaplan–Meier curves were used to assess the risk of BCR. Proportional hazards multivariable regression models were composed to determine prognostic factors for BCR. SPSS version 12.0.1 for Windows was used for all statistical analysis.
Results
Patient and tumor characteristics are summarized in Table 1. The age of the patients at time of RP was 62.7 years (IQR 58.4–66.5 years) and they had a median Gleason score of 6. In the specimens, a median MTD of 24.0 mm (IQR 16.0–32.0 mm) was found. Patients were followed for a median period of 39.5 months (IQR 17.1–67.3 months). Overall, 114 (21%) patients developed BCR after RP. The median time to PSA recurrence was 20.3 months (IQR 10.3–46.9). The overall 5-year Kaplan–Meier risk of BCR was 25% (95% CI = 20.4–29.6) for the whole group. For the high-risk group and low/intermediate risk, these were 27.4 (95% CI = 22.2–32.6) and 10% (95% CI = 2.2–17.8), respectively. Totally, 432 of the 542 patients fulfilled the criteria for high-risk localized PC, 72 patients were low/intermediate risk, and 38 patients never reached non-measurable PSA levels.
Associations between MTD and pathological characteristics are summarized in Table 2. Overall we found 359 organ-confined (T2) tumors, 116 extracapsular extended tumors (T3a), and 52 tumors invaded into seminal vesicles (T3b). Half of all the patients (n = 271) had positive surgical margins and eight patients had lymph node involvement. Pre-operative PSA levels ranged from 0.1–87.2 ng/ml (median 8.0 ng/ml). Kruskall–Wallis tests or Mann–Whitney U test revealed that MTD was significantly associated with pathological stage (P < 0.001), Gleason score (P < 0.001), margin status (P < 0.001), number of tumors (P < 0.001) and pre-operative PSA levels (P < 0.001). Positive surgical margins were associated with a larger MTD compared to negative surgical margins. Single tumors were also found to have a larger MTD than multiple tumors. Extracapsular extension (T3a) and invasion to seminal vesicles (T3b) were both associated with larger maximal tumor diameters compared to those of organ-confined (T2) tumors. MTD in the high-risk group was significantly higher then in the low/intermediate risk group (median 24.5 vs.14.0 mm, P < 0.001). We also calculated the correlation coefficient for MTD and all other factors. We found that MTD was significantly correlated to index TV (=largest tumor) (r = 0.60, P < 0.001) and total TV (r = 0.61, P < 0.001) in the total group. In the high-risk group, these correlations were weaker. In addition, significant correlations with MTD were found for Gleason score (r = 0.17, P < 0.001), the number of tumors (r = −0.21, P < 0.001) and pre-operative PSA levels (r = 0.38, P < 0.001).
Univariable analyses of MTD as a continuous variable revealed that it is weakly associated with risk of BCR (HR = 1.02 per mm increase, 95% CI = 1.00–1.04, P = 0.024). In the high-risk group, this association was lost (HR = 1.01 per mm increase, 95%CI = 0.99–1.03, P = 0.18).
In the total group TV, pre-operative PSA levels, pathological stage, and Gleason score were associated with risk of BCR, as presented in Table 3. Proportional hazards multivariable regression models were composed to determine significant independent prognostic factors (Table 3). Positive surgical margins, higher Gleason score, advanced pathological stage, and multiple tumors were the main prognostic factors for BCR. Neither MTD nor tumor volume turned out to be significant in either group.
Discussion
Prediction of outcome after RP in patients with localized PC is important to tailor follow up, and for example, to consider adjuvant therapy in patients at high risk for recurrence. Several studies have assessed the value of morphological and clinical variables in this respect. Tumor stage, Gleason score, and pre-operative PSA levels are obvious parameters, and also used to define patients at high-risk for recurrence [2]. MTD, as easy to determine surrogate for tumor volume [12], is another potential variable considered as a potential predictor of BCR. In our study of 542 patients, we found that MTD correlates with index TV, total TV, pathological stage, Gleason score, number of tumors, and pre-operative PSA levels irrespective of risk classification. However, MTD was not an independent predictor of BCR in the total or in the high-risk subgroup. For the whole group, these results are in contrast with previously reported findings.
One of the first studies addressing the association of MTD and BCR was by Renshaw et al. [3]. In this study, with 57 patients undergoing RP, it was found that MTD was strongly correlated with total TV. In this small series, a high MTD was associated with a slight increased risk for BCR (HR 1.12) that was marginally significant. Their subsequent study was a larger cohort of 434 patients. The median MTD was 13 mm. The median follow-up was 12.9 months and 27.2% of patients had PSA failure. This study confirmed that MTD is an independent predictor of BCR, even after adjustment for other risk factors [11].
Their next study by Dvorak et al. [15] had an even larger cohort (781 patients) and longer median follow-up (64.8 months). MTD remained the same with a median of 13 mm, and BCR in 31% of the patients. Again they found that MTD was significantly associated with time to BCR (HR = 1.04, P = 0.004) adjusted for pre-op PSA, Gleason score, and pathological stage. But when surgical margin status was added, MTD lost statistical significance (P = 0.07). One important feature of these studies is that the RP specimens were not uniformly processed. Therefore, we have to be taking into account the variation in evaluation of the prognostic factors. Another difference with our results is that the median MTD in these three studies (13 mm) are less than in our study (24 mm). This might be explained by the way we determine our MTD. Renshaw et al. limited their measurements to the single focus of tumor that could be demonstrated on one slide (slice-MTD). We, on the other hand, take the largest diameter of the largest tumor after reconstruction (real-MTD). To see if we could explain this difference, we also measured the slice-MTD in a part of our cohort. In 250 consecutive cases from 2000 to 2005, we found a median slice-MTD of 19 mm and a median real-MTD of 24 mm. This strongly suggests that part of the difference in MTD is caused by the different way MTD is measured. The difference that still exists could be explained by the fact that Renshaw et al. used partially submitted prostates, thus missing part of the prostates for evaluation. The only study where they did use uniformly processed specimens is by Eichelberger et al., but this study has a very short follow-up. They analyzed the prognostic value of MTD in a cohort of 364 patients treated with RP, and found a mean MTD of 17.3 mm and biochemical recurrence in 12% of the patients [16]. In this study with a median follow-up of only 12 months, they found that MTD was a significant predictor of BCR, adjusted for Gleason score and surgical margin status (HR = 1.70, P = 0.011). In our study we found a median time to BCR of 20.3 months, hence a follow-up of 12 months seems not long enough to investigate BCR.
Another striking difference is the percentage of positive surgical margins. In our study this is relatively high (50%) compared to other studies (Renshaw et al. 31 and 26%, Eichelberger et al. 23%, Dvorak et al. 27%). Our median BCR rate (21% after 39.5 months), however, was comparable to the median of these studies [Renshaw 27% (22.5 months), Eichelberger 12% (12 months), Dvorak 31% (64.8 months)]. An explanation for the high number of positive surgical margins, next to surgical techniques, is that by evaluating only partially included prostates you miss positive surgical margins.
The difference between our study and studies like Eichelberger et al, in which the entire prostate was histologically examined using whole mount sections, are less easily explained, but here the interval at which the prostates were sliced is higher (mean 7.8 mm according to the used protocol described by Qian et al. [17], personal communication). From this, we can conclude that MTD is not an independent prognostic marker if surgical resection margins are accurately documented.
Finally, none of the mentioned studies separately looked at the group where prediction might be of most importance, the high-risk group. As clearly indicated, in this group, neither MTD nor TV is of any significant value.
In all, we do not advice to use MTD as a prognostic factor for BCR, since after proper follow-up and with adequate pathological techniques it does not add anything to other readily available prognostic factors. Moreover, in the high-risk group, TV is not an independent prognostic factor at all.
Conclusions
The results of our study suggest that MTD is positively correlated to total TV, as can be expected. MTD was weakly associated with risk of BCR and this association was lost in the high-risk group. If adjusted for positive surgical margins, higher Gleason score, advanced pathological stage, and multiple tumors, MTD did not provide additional information.
Reference
Han M, Partin AW, Pound CR, Epstein JI, Walsh PC (2001) Long-term biochemical disease-free and cancer-specific survival following anatomic radical retropubic prostatectomy. The 15-year Johns Hopkins experience. Urol Clin North Am 28:555–565
D’Amico AV, Whittington R, Malkowicz SB et al (2002) Biochemical outcome after radical prostatectomy or external beam radiation therapy for patients with clinically localized prostate carcinoma in the prostate specific antigen era. Cancer 95:281–286
Renshaw AA, Richie JP, Loughlin KR, Jiroutek M, Chung A, D’Amico AV (1998) The greatest dimension of prostate carcinoma is a simple, inexpensive predictor of prostate specific antigen failure in radical prostatectomy specimens. Cancer 83:748–752
Epstein JI, Carmichael M, Partin AW, Walsh PC (1993) Is tumor volume an independent predictor of progression following radical prostatectomy? A multivariate analysis of 185 clinical stage B adenocarcinomas of the prostate with 5 years of followup. J Urol 149:1478–1481
Salomon L, Levrel O, Anastasiadis AG et al (2003) Prognostic significance of tumor volume after radical prostatectomy: a multivariate analysis of pathological prognostic factors. Eur Urol 43:39–44
Stamey TA, McNeal JE, Yemoto CM, Sigal BM, Johnstone IM (1999) Biological determinants of cancer progression in men with prostate cancer. JAMA: 281:1395–1400
Bostwick DG, Graham SD Jr, Napalkov P et al (1993) Staging of early prostate cancer: a proposed tumor volume-based prognostic index. Urology 41:403–411
Nelson BA, Shappell SB, Chang SS et al (2006) Tumour volume is an independent predictor of prostate-specific antigen recurrence in patients undergoing radical prostatectomy for clinically localized prostate cancer. BJU Int 97:1169–1172
Wise AM, Stamey TA, McNeal JE, Clayton JL (2002) Morphologic and clinical significance of multifocal prostate cancers in radical prostatectomy specimens. Urology 60:264–269
Humphrey PA, Vollmer RT (1997) Percentage carcinoma as a measure of prostatic tumor size in radical prostatectomy tissues. Mod Pathol 10:326–333
Renshaw AA, Richie JP, Loughlin KR, Jiroutek M, Chung A, D’Amico AV (1999) Maximum diameter of prostatic carcinoma is a simple, inexpensive, and independent predictor of prostate-specific antigen failure in radical prostatectomy specimens. Validation in a cohort of 434 patients. Am J Clin Pathol 111:641–644
Eichelberger LE, Koch MO, Daggy JK, Ulbright TM, Eble JN, Cheng L (2003) Predicting tumor volume in radical prostatectomy specimens from patients with prostate cancer. Am J Clin Pathol 120:386–391
Ruijter ET, Miller GJ, Aalders TW et al (1997) Rapid microwave-stimulated fixation of entire prostatectomy specimens. Biomed-II MPC Study Group. J Pathol 183:369–375
Greene FL, Page DL, Fleming ID et al (2002) AJCC cancer staging manual, 6th edn. Springer, New York
Dvorak T, Chen MH, Renshaw AA, Loffredo M, Richie JP, D’Amico AV (2005) Maximal tumor diameter and the risk of PSA failure in men with specimen-confined prostate cancer. Urology 66:1024–1028
Eichelberger LE, Koch MO, Eble JN, Ulbright TM, Juliar BE, Cheng L (2005) Maximum tumor diameter is an independent predictor of prostate-specific antigen recurrence in prostate cancer. Mod Pathol 18:886–890
Qian J, Wollan P, Bostwick DG (1997) The extent and multicentricity of high-grade prostatic intraepithelial neoplasia in clinically localized prostatic adenocarcinoma. Hum Pathol: 28:143–148
Conflict of interest statement
There is no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
van Oort, I.M., Witjes, J.A., Kok, D.E.G. et al. Maximum tumor diameter is not an independent prognostic factor in high-risk localized prostate cancer. World J Urol 26, 237–241 (2008). https://doi.org/10.1007/s00345-008-0242-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00345-008-0242-7