Abstract
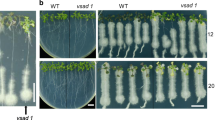
Anthocyanins are important natural pigments in plants, and they function not only in antioxidation but also in plant stress responses, pollination, and seed propagation. The anthocyanin-more (am) mutant described in our study is a naturally occurring mutant of Brassica napus cultivar Zhongshuang 11. Compared with the wild type (WT), the mutant produced more anthocyanins at the seedling, bolting, and maturity stages, showing purple color in different parts of the plant. The protein expression profiles of the mutant and WT seedling leaves under natural growth conditions were analyzed using proteomics. A total of 32 proteins that exhibited at least 1.5-fold difference in expression between the WT and mutant plants were successfully identified. The proteins were mainly involved in defense/stress/detoxification and photosynthesis. In particular, the defense/stress/detoxification response and anthocyanin synthesis-related proteins were significantly affected in the am mutant. Moreover, we found the am mutant can enhance the resistance to Sclerotinia sclerotiorum compared with the WT. This enhanced disease resistance may result from the increased anthocyanin content, which is also related to antimicrobial activities and inhibiting fungal growth, and the rapid accumulation of reactive oxygen species that are involved in programmed cell death in plants. These results provide a basis for studying the mutant’s molecular mechanism and increase the information available regarding the growth and development of rapeseed under stress conditions.








Similar content being viewed by others
References
Asem ID, Imotomba RK, Mazumder PB, Laishram JM (2015) Anthocyanin content in the black scented rice (Chakhao): its impact on human health and plant defense. Symbiosis 66:47–54
Baxter A, Mittler R, Suzuki N (2014) ROS as key players in plant stress signalling. J Exp Bot 65:1229–1240
Bi H, Guo M, Wang J, Qu Y, Du W, Zhang K (2018) Transcriptome analysis reveals anthocyanin acts as a protectant in Begonia semperflorens under low temperature. Acta Physiol Plant 40:10
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Calla B, Vuong T, Radwan O, Hartman GL, Clough SJ (2009) Gene expression profiling soybean stem tissue early response to Sclerotinia sclerotiorum and in silico mapping in relation to resistance markers. Plant Genome 2:149–166
Chen W, Wan S, Shen L, Zhou Y, Huang C, Chu P, Guan R (2018) Histological, physiological, and comparative proteomic analyses provide insights into leaf rolling in Brassica napus. J Proteome Res 17:1761–1772
Chiu LW, Zhou X, Burke S, Wu X, Prior RL, Li L (2010) The purple cauliflower arises from activation of a MYB transcription factor. Plant Physiol 154:1470–1480
Cutuli B, Lemanski C, Fourquet A, Lafontan BD, Giard S, Lancrenon S, Meunier A, Pioud-Martigny R, Campana F, Marsiglia H (2010) Environmental significance of anthocyanins in plant stress responses. Photochem Photobiol 70:1–9
De P-TS, Moreno DA, Garcã-A-Viguera C (2010) Flavanols and anthocyanins in cardiovascular health: a review of current evidence. Int J Mol Sci 11:1679–1703
Dixon RA, Achnine L, Kota P, Liu CJ, Reddy MS, Wang L (2010) The phenylpropanoid pathway and plant defence-a genomics perspective. Mol Plant Pathol 3:371–390
Duan S, Wang J, Gao C, Jin C, Dong L, Peng D, Du G, Li Y, Chen M (2018) Functional characterization of a heterologously expressed Brassica napus WRKY41-1 transcription factor in regulating anthocyanin biosynthesis in Arabidopsis thaliana. Plant Sci 268:47–53
Fan W, Cui W, Li X, Chen S, Liu G, Shen S (2011) Proteomics analysis of rice seedling responses to ovine saliva. J Plant Physiol 168:500–509
Ghosh D, Konishi T (2007) Anthocyanins and anthocyanin-rich extracts: role in diabetes and eye function. Asia Pac J Clin Nutr 16:200–208
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Gonzalez A, Zhao M, Leavitt JM, Lloyd AM (2008) Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/Myb transcriptional complex in Arabidopsis seedlings. Plant J 53:814–827
Grit R, Takayuki T, Fumio M, Kazuki S, Wolf-Rüdiger S (2009) Members of the LBD family of transcription factors repress anthocyanin synthesis and affect additional nitrogen responses in Arabidopsis. Plant Cell 21:3567–3584
Hayashi K, Matsumoto S, Tsukazaki H, Kondo T, Kubo N, Hirai M (2010) Mapping of a novel locus regulating anthocyanin pigmentation in Brassica rapa. Breed Sci 60:76–80
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Jain PK, Kharya MD, Gajbhiye A, Sara UVS, Sharma VK (2008) Flavonoids as nutraceuticals. A review. Herba Pol 7:1089–1099
Juvany M, Müller M, Munnébosch S (2013) Photo-oxidative stress in emerging and senescing leaves: a mirror image? J Exp Bot 64:3087–3098
Kim YO, Kim JS, Kang H (2005) Cold-inducible zinc finger-containing glycine-rich RNA-binding protein contributes to the enhancement of freezing tolerance in Arabidopsis thaliana. Plant J 42:890–900
Kim YB, Park SY, Thwe AA, Seo JM, Suzuki T, Kim SJ, Kim JK, Park SU (2013) Metabolomic analysis and differential expression of anthocyanin biosynthetic genes in white- and red-flowered buckwheat cultivars (Fagopyrum esculentum). J Agric Food Chem 61:10525–10533
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Li X, Gao MJ, Pan HY, Cui DJ, Gruber MY (2010) Purple canola: Arabidopsis PAP1 increases antioxidants and phenolics in Brassica napus leaves. J Agric Food Chem 58:1639
Li H, Zhu L, Yuan G, Heng S, Yi B, Ma C, Shen J, Tu J, Fu T, Wen J (2016) Fine mapping and candidate gene analysis of an anthocyanin-rich gene, BnaA.PL1, conferring purple leaves in Brassica napus L. Mol Genet Genom 291:1–12
Liang Y, Srivastava S, Rahman MH, Strelkov SE, Kav NN (2008) Proteome changes in leaves of Brassica napus L. as a result of Sclerotinia sclerotiorum challenge. J Agric Food Chem 56:1963–1976
Liang L, Wu X, Zhao T, Zhao J, Fang L, Ye Z, Mao G, Yang L (2012) In vitro bioaccessibility and antioxidant activity of anthocyanins from mulberry (Morus atropurpurea Roxb.) following simulated gastro-intestinal digestion. Food Res Int 46:76–82
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408
Lv JL (2008) Primary mapping of leaf color gene in Brassica napus. Ph. D. Dissertation (in Chinese), Huazhong Agricultural University
Mehrtens F, Kranz H, Bednarek P, Weisshaar B (2005) The Arabidopsis transcription factor MYB12 is a flavonol-specific regulator of phenylpropanoid biosynthesis. Plant Physiol 138:1083–1096
Petrussa E, Braidot E, Zancani M, Peresson C, Bertolini A, Patui S, Vianello A (2013) Plant flavonoids–biosynthesis, transport and involvement in stress responses. Int J Mol Sci 14:14950–14973
Pojer E, Mattivi F, Johnson D, Stockley CS (2013) The case for anthocyanin consumption to promote human health: a review. Compr Rev Food Sci Food 12:483–508
Raines CA (2010) Transgenic approaches to manipulate the environmental responses of the C3 carbon fixation cycle. Plant, Cell Environ 29:331–339
Ritchie RJ (2006) Consistent sets of spectrophotometric chlorophyll equations for acetone, methanol and ethanol solvents. Photosynth Res 89:27–41
Schaefer H, Rolshausen G (2010) Plants on red alert: do insects pay attention? BioEssays 28:65–71
Song TH, Han OK, Yangkil K, Taeil P, Kihun P, Keejong K (2011) Effect of top dressing and harvest time on growth, feed value, and anthocyanin content of colored barley. Korean J Crop Sci 56:159–166
Tanaka Y, Ohmiya A (2008) Seeing is believing: engineering anthocyanin and carotenoid biosynthetic pathways. Curr Opin Biotechnol 19:190–197
Thangella PAV, Pasumarti SNBS, Pullakhandam R, Geereddy BR, Daggu MR (2018) Differential expression of leaf proteins in four cultivars of peanut (Arachis hypogaea L.) under water stress. Biotech 8:157
Wan L, Zha W, Cheng X, Liu C, Lv L, Liu C, Wang Z, Du B, Chen R, Zhu L, He G (2011) A rice β-1,3-glucanase gene Osg1 is required for callose degradation in pollen development. Planta 233:309–323
Wang W, Zhang D, Yu S, Jin L, Dan W, Zhang F, Yu Y, Zhao X, Lu G, Su T (2014) Mapping the Br Pur gene for purple leaf color on linkage group A03 of Brassica rapa. Euphytica 199:293–302
Wang Z, Fang H, Chen Y, Chen K, Li G, Gu S, Tan X (2015) Overexpression of BnWRKY33 in oilseed rape enhances resistance to Sclerotinia sclerotiorum. Mol Plant Pathol 15:677–689
Wang JL, Tang MQ, Chen S, Zheng XF, Mo HX, Li SJ, Wang Z, Zhu KM, Ding LN, Liu SY (2017) Down-regulation of BnDA1, whose gene locus is associated with the seeds weight, improves the seeds weight and organ size in Brassica napus. Plant Biotechnol J 15:1024–1033
Wang Z, Yang C, Chen H, Wang P, Wang P, Song C, Zhang X, Wang D (2018) Multi-gene co-expression can improve comprehensive resistance to multiple abiotic stresses in Brassica napus L. Plant Sci 274:410–419
Wang Z, Bao LL, Zhao FY, Tang MQ, Chen T, Li YM, Wang BX, Fu BZ, Fang HD, Li GY, Cao J, Ding LN, Zhu KM, Liu SY, Tan XL (2019) BnaMPK3 is a key regulator of defense responses to the devastating plant pathogen Sclerotinia sclerotiorum in oilseed rape. Front Plant Sci 10:18
Wu X, Liang L, Zou Y, Zhao T, Zhao J, Li F, Yang L (2011) Aqueous two-phase extraction, identification and antioxidant activity of anthocyanins from mulberry (Morus atropurpurea Roxb.). Food Chem 129:443–453
Wu XJ, Hu BC, Chen FX, Li QS, Hou SM, Fei WX, Huang XR, Jianf YF (2014) Breeding of the temporary all maintainer with purple leaf in oilseed rape (Brassica napus. L). Genet Breed 23:21–28
Yan LY, Pang BP, Zhou XR, Zhang CQ (2008) Relationship between host plant preference of Liriomyza huidobrensis for different Phaseolus vulgaris varieties and plant compound contents. Sci Agric Sin 41:713–719
Yang YH, Li D, Xia HC, Zhu KM, Liu XY, Chen KP (2013) Comparative proteomic analysis of rice stripe virus (RSV)-resistant and -susceptible rice cultivars. Aust J Crop Sci 7:588–593
Yao Y, Sun H, Xu F, Zhang X, Liu S (2011) Comparative proteome analysis of metabolic changes by low phosphorus stress in two Brassica napus genotypes. Planta 233:523–537
Yao P, Zhao H, Luo X, Gao F, Li C, Yao H, Chen H, Park S-U, Wu Q (2017) Fagopyrum tataricum FtWD40 functions as a positive regulator of anthocyanin biosynthesis in transgenic tobacco. J Plant Growth Regul 36:755–765
Yıldız M, Akçalı N, Terzi H (2015) Proteomic and biochemical responses of canola (Brassica napus L.) exposed to salinity stress and exogenous lipoic acid. J Plant Physiol 179:90–99
Yuan Y, Chiu LW, Li L (2009) Transcriptional regulation of anthocyanin biosynthesis in red cabbage. Planta 230:1141
Zhan LY, Lin YZ (2014) Anthocyanin accumulation and transcriptional regulation of anthocyanin biosynthesis in Purple Bok Choy (Brassicarapa var. chinensis). J Agric Food Chem 62:12366–12376
Zhang BX, Zhu YM, Lai YC, Hu XM, Li W, Li W, Bi YD, Xiao JL, Zhang LL (2012) Research progress of plant chalcone synthase (CHS) and its gene. J Anhui Agric Sci 40:10376–10379
Zhang L, Yu YY, Liu Y, Huang XQ, Zhou XQ, Liu HY (2015) Pathogenicity differentiation of Sclerotinia sclerotiorum from the same strains derived from different rapeseed inoculums. Chin J Oil Crop Sci 37:201–205
Zhao Z, Xiao L, Xu L, Xing X, Tang G, Du D (2017) Fine mapping the BjPl1 gene for purple leaf color in B2 of Brassica juncea L. through comparative mapping and whole-genome re-sequencing. Euphytica 213:80
Zhu Z, Lu Y (2016) Plant color mutants and the anthocyanin pathway. Chin Bull Bot 51:107–119
Acknowledgements
This work was supported by National Key Research and Development Program of China (2016YFD0100305; 2016YFD0101904) and National Natural Science Foundation of China (31471527; 31271760).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no competing interests exist.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, R., Ding, LN., Li, M. et al. Characterization of a Rapeseed Anthocyanin-More Mutant with Enhanced Resistance to Sclerotinia sclerotiorum. J Plant Growth Regul 39, 703–716 (2020). https://doi.org/10.1007/s00344-019-10011-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-019-10011-4