Abstract
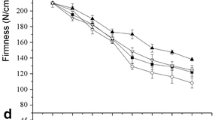
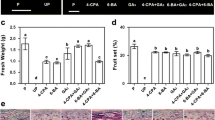
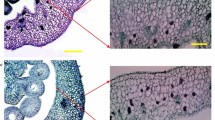
This study explores the unique growth-regulatory roles of two naturally occurring auxins, indole-3-acetic acid (IAA) and 4-chloroindole-3-acetic acid (4-Cl-IAA), and their interactions with gibberellin (GA) during early pea (Pisum sativum L.) fruit development. We have previously shown that 4-Cl-IAA can replace the seed requirement in pea pericarp growth (length and fresh weight), whereas IAA had no effect or was inhibitory. When applied simultaneously, gibberellin (GA3 or GA1) and 4-Cl-IAA had a synergistic effect on pericarp growth. In the present study, we found that simultaneous application of IAA and GA3 to deseeded pericarps inhibited GA3-stimulated growth. The inhibitory effect of IAA on GA-stimulated growth was mimicked by treatment with ethephon (ethylene releasing agent), and the inhibitory effects of IAA and ethylene on GA-mediated growth were reversed by silver thiosulfate (STS), an ethylene action inhibitor. Although pretreatment with STS could retard senescence of IAA-treated pericarps, STS pretreatment did not lead to IAA-induced pericarp growth. Although 4-Cl-IAA stimulated growth whereas IAA was ineffective, both auxins induced similar levels of ethylene evolution. However, only 4-Cl-IAA-stimulated growth was insensitive to the effects of ethylene. Gibberellin treatment did not influence the amount of ethylene released from pericarps in the presence or absence of either auxin. We propose a growth regulatory role for 4-Cl-IAA through induction of GA biosynthesis and inhibition of ethylene action. Additionally, ethylene (IAA-induced or IAA-independent) may inhibit GA responses under physiological conditions that limit fruit growth.






Similar content being viewed by others
References
Achard P, Vriezen WH, Van Der Straeten D, Harberd NP. 2003. Ethylene regulates Arabidopsis development via the modulation of DELLA protein growth repressor function. Plant Cell 15:2816–2825
Ahmad A, Andersen A, Engvild K. 1987. Rooting, growth and ethylene evolution of pea cuttings in response to chloroindole auxins. Physiol Plant 69:137–140
Burg SP, Burg EA. 1968. “Auxin stimulated ethylene formation: its relationship to auxin inhibited growth, root geotropism and other plant processes” In: Wightman F, Setterfield G (eds.), Biochemistry and Physiology of Plant Growth Substances, Ottawa, Canada, The Runge Press, pp 1275–1294
Eeuwens CJ, Schwabe WW. 1975. Seed and pod wall development in Pisum sativum L. in relation to extracted and applied hormones. J Exp Bot 26:1–14
Engvild KC, Egsgaard H, Larsen E. 1980. Determination of 4-chloroindole-3-acetic acid methyl-ester in Lathyrus, Vicia and Pisum by gas chromatography-mass spectrometry. Physiol Plant 48:499–503
Engvild KC, Egsgaard H, Larsen E. 1981. Determination of 4-chloroindoleacetic acid methyl-ester in Vicieae species by gas chromatography-mass spectrometry. Physiol Plant 53:79–81
Fu XD, Harberd NP. 2003. Auxin promotes Arabidopsis root growth by modulating gibberellin response. Nature 421:740–743
Garcia-Martinez JL, Santes CM, Croker SJ, Hedden P. 1991. Identification, quantitation and distribution of gibberellins in fruits of Pisum sativum L. cv. Alaska during pod development. Planta 184:523–530
Gomez-Gomez L, Carrasco P. 1996. Hormonal regulation of S-adenosylmethionine synthase transcripts in pea ovaries. Plant Mol Biol 30:821–832
Lincoln C, Britton JH, Estelle M. 1990. Growth and development of the axr1 mutants of Arabidopsis. Plant Cell 2:1071–1080
Luschnig C, Gaxiola RA, Grisafi P, Fink GR. 1998. EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Genes Dev 12:2175–2187
Magnus V, Ozga JA, Reinecke DM, Pierson GL, Larue TA, and others. 1997. 4-Chloroindole-3-acetic and indole-3-acetic acids in Pisum sativum. Phytochemistry 46:675–681
Ngo P, Ozga JA, Reinecke DM. 2002. Specificity of auxin regulation of gibberellin 20-oxidase gene expression in pea pericarp. Plant Mol Biol 49:439–448
Orzáez D, Blay R, Granell A. 1999. Programme of senescence in petals and carpels of Pisum sativum L. flowers and its control by ethylene. Planta 208:220–226
Orzáez D, Granell A. 1997. DNA fragmentation is regulated by ethylene during carpel senescence in Pisum sativum. Plant J 11:137–144
Ozga JA, Brenner ML, Reinecke DM. 1992. Seed effects on gibberellin metabolism in pea pericarp. Plant Physiol 100:88–94
Ozga JA, Reinecke DM. 1999. Interaction of 4-chloroindole-3-acetic acid and gibberellins in early pea fruit development. J Plant Growth Regul 27:33–38
Ozga JA, Reinecke DM. 2003. Hormonal interactions in fruit development. J Plant Growth Regul 22: 73–81
Ozga JA, van Huizen R, Reinecke DM. 2002. Hormone and seed-specific regulation of pea fruit growth. Plant Physiol 128:1379–1389
Ozga JA, Yu J, Reinecke DM. 2003. Pollination-, development-, and auxin-specific regulation of gibberellin 3β-hydroxylase gene expression in pea fruit and seeds. Plant Physiol 131:1137–1146
Peck SC, Kende H. 1995. Sequential induction of the ethylene biosynthetic enzymes by indole-3-acetic acid in etiolated peas. Plant Mol Biol 28:293–301
Peck SC, Kende H. 1998. Differential regulation of genes encoding 1-aminocyclopropane-1-carboxylate (ACC) synthase in etiolated pea seedlings: effects of indole-3-acetic acid, wounding, and ethylene. Plant Mol Biol 38:977–982
Pless T, Bottger M, Hedden P, Graebe J. 1984. Occurrence of 4-Cl-indoleacetic acid in broad beans and correlation of its levels with seed development. Plant Physiol 74:320–323
Pharis RP, King RW. 1985. Gibberellins and reproductive development in seed plants. Annu Rev Plant Physiol Plant Mol Biol 36:517–568
Reinecke DM, Ozga JA, Magnus V. 1995. Effect of halogen substitution of indole-3-acetic-acid on biological activity in pea fruit. Phytochemistry 40:1361–1366
Richards DE, King KE, Ait-ali T, Harberd NP. 2001. How gibberellin regulates plant growth and development: a molecular genetic analysis of gibberellin signaling. Annu Rev Plant Physiol Plant Mol Biol 52:67–88
Rodrigo MJ, Garcia-Martinez JL, Santes CM, Gaskin P, Hedden P. 1997. The role of gibberellins A1 and A3 in fruit growth of Pisum sativum L. and the identification of gibberellins A4 and A7 in young seeds. Planta 201:446–455
Sponsel VM. 1995. “The biosynthesis and metabolism of gibberellins in higher plants” In: Davies PJ (ed.), Plant Hormones: Physiology, Biochemistry and Molecular Biology, 2nd Editon, Dordrecht, The Netherlands, Kluwer Academic Publishers, pp 66–97
van Huizen R, Ozga JA, Reinecke DM. 1997. Seed and hormonal regulation of gibberellin 20-oxidase expression in pea pericarp. Plant Physiol 115:123–128
van Huizen R, Ozga JA, Reinecke DM, Twitchin B, Mander LN. 1995. Seed and 4-chloroindole-3-acetic acid regulation of gibberellin metabolism in pea pericarp. Plant Physiol 109:1213–1217
Warner HL, Leopold AC. 1969. Ethylene evolution from 2-chloroethylphosphonic acid. Plant Physiol 44:156–158
Acknowledgments
This work was funded by a Natural Science and Engineering Research Council of Canada Discovery Grant (OGPO138166) to J. Ozga; and the SM Blair scholarship to M. Johnstone.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Johnstone, M.M.G., Reinecke, D.M. & Ozga, J.A. The Auxins IAA and 4-Cl-IAA Differentially Modify Gibberellin Action via Ethylene Response in Developing Pea Fruit. J Plant Growth Regul 24, 214–225 (2005). https://doi.org/10.1007/s00344-005-0035-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-005-0035-9