Abstract
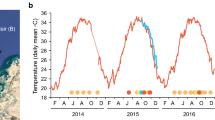
While links between heat stress and coral bleaching are clear and predictive tools for bleaching risk are well advanced, links between heat stress and outbreaks of coral diseases are less well understood. In this study, the effects of accumulated heat stress on tagged colonies of tabular Acropora were monitored over the 2017 austral summer at Beaver Reef, which is located in the central region of the Great Barrier Reef. The initial surveys in midsummer (21 February) coincided with an accumulated heat stress metric of 4.5 °C-weeks, and documented high coral cover (74.0 ± 6.5%), extensive bleaching (71% of all corals displayed bleaching signs) and an outbreak of white syndromes (WSs) (31% of tabular acroporid corals displayed white syndrome signs). Repeat assessments of the impacts of bleaching and disease on these corals provided real-time information to reef managers by tracking the unfolding reef health incident on 100 colonies of Acropora hyacinthus (Dana, 1846), tagged in mid-March and surveyed intermittently until late October 2017. Heat stress increased rapidly on Beaver Reef, peaking at 8.3 °C-weeks on 31 March, which coincided with the highest prevalence of WS recorded in the study. Of the 85 tagged colonies surviving on 31 March, 41 (~ 48%) displayed WS signs, indicating a link between heat stress and WS. When re-surveyed at eight months (24 October), 68 of 100 tagged colonies had suffered whole-colony mortality and only four colonies had not displayed signs of bleaching or disease (WS) in any of our surveys. Overall, coral cover on Beaver Reef was reduced by more than half to 31.0 ± 11.2%. Significant tissue loss due to severe bleaching was observed with up to 20 times greater tissue loss on severely bleached colonies (i.e. categorised as > 50% bleached) compared to mildly/moderately bleached colonies (< 50% bleached) at the heat stress peak (31 March). This suggests that for Acropora hyacinthus, a threshold of 50% colony bleaching is a good indicator that substantial mortality at both the colony and population level is likely to follow a heat stress event. Across all levels of bleaching, colonies displaying WS signs exhibited up to seven times greater tissue loss than bleached-only colonies. WS caused a threefold increase in accumulated tissue loss (69.6 ± 10.5% tissue lost) in the mildly bleached category, suggesting that disease exacerbated mortality in bleached corals and contributed significantly to the substantial loss of corals on the GBR in 2017.






Similar content being viewed by others
References
Anthony KRN, Connolly SR, Hoegh-Guldberg O (2007) Bleaching, energetics, and coral mortality risk: Effects of temperature, light, and sediment regime. Limnol Oceanogr 52:716–726
Anthony KRN, Hoogenboom MO, Maynard JA, Grottoli AG, Middlebrook R (2009) Energetics approach to predicting mortality risk from environmental stress: A case study of coral bleaching. Funct Ecol 23:539–550
Baird AH, Marshall PA (2002) Mortality, growth and reproduction in scleractinian corals following bleaching on the Great Barrier Reef. Mar Ecol Prog Ser 237:133–141
Baker AC, Glynn PW, Riegl B (2008) Climate change and coral reef bleaching: An ecological assessment of long-term impacts, recovery trends and future outlook. Estuar Coast Shelf Sci 80:1–37
Ban SS, Graham NAJ, Connolly SR (2013) Relationships between temperature, bleaching and white syndrome on the Great Barrier Reef. Coral Reefs 32:1–12
Beeden RJ, Turner MA, Dryden J, Merida F, Goudkamp K, Malone C, Marshall PA, Birtles A, Maynard JA (2014) Rapid survey protocol that provides dynamic information on reef condition to managers of the Great Barrier Reef. Environ Monit Assess 186:8527–8540
Berkelmans R, van Oppen MJH (2006) The role of zooxanthellae in the thermal tolerance of corals: A “nugget of hope” for coral reefs in an era of climate change. Proc R Soc B Biol Sci 273:2305–2312
Bourne DG, Ainsworth TD, Pollock FJ, Willis BL (2015) Towards a better understanding of white syndromes and their causes on Indo-Pacific coral reefs. Coral Reefs 34:233–242
Brandt M, McManus JW (2009) Disease incidence is related to bleaching extent in reef-building corals. Ecology 90:2859–2867
Bruno JF, Selig ER, Casey KS, Page CA, Willis BL, Harvell CD, Sweatman H, Melendy AM (2007) Thermal stress and coral cover as drivers of coral disease outbreaks. PLoS Biol 5:e124
Darling ES, Graham NAJ, Januchowski-Hartley FA, Nash KL, Pratchett MS, Wilson SK (2017) Relationships between structural complexity, coral traits, and reef fish assemblages. Coral Reefs 36:561–575
Done TJ, DeVantier LM, Turak E, Fisk DA, Wakeford M, van Woesik R (2010) Coral growth on three reefs: development of recovery benchmarks using a space for time approach. Coral Reefs 29:815–833
Eakin CM, Morgan JA, Heron SF, Smith TB, Liu G, Alvarez-Filip L, Baca B, Bartels E, Bastidas C, Bouchon C, Brandt M, Bruckner AW, Bunkley-Williams L, Cameron A, Causey BD, Chiappone M, Christensen TRL, Crabbe MJC, Day O, de la Guardia E, Díaz-Pulido G, DiResta D, Gil-Agudelo DL, Gilliam DS, Ginsburg RN, Gore S, Guzmán HM, Hendee JC, Hernández-Delgado EA, Husain E, Jeffrey CFG, Jones RJ, Jordán-Dahlgren E, Kaufman LS, Kline DI, Kramer PA, Lang JC, Lirman D, Mallela J, Manfrino C, Maréchal J-P, Marks K, Mihaly J, Miller WJ, Mueller EM, Muller EM, Orozco Toro CA, Oxenford HA, Ponce-Taylor D, Quinn N, Ritchie KB, Rodríguez S, Ramírez AR, Romano S, Samhouri JF, Sánchez JA, Schmahl GP, Shank BV, Skirving WJ, Steiner SCC, Villamizar E, Walsh SM, Walter C, Weil E, Williams EH, Roberson KW, Yusuf Y (2010) Caribbean Corals in Crisis: Record Thermal Stress, Bleaching, and Mortality in 2005. PLoS One 5:e13969
Frölicher TL, Fischer EM, Gruber N (2018) Marine heatwaves under global warming. Nature 560:360–364
GBRMPA (2013) Reef health incident response system. Great Barrier Reef Marine Park Authority, Townsville
GBRMPA (2017a) Reef health update. Great Barrier Reef Marine Park Authority, Townsville
GBRMPA (2017b) Final report: 2016 coral bleaching event on the Great Barrier Reef. Great Barrier Reef Marine Park Authority, Townsville
Graham NAJ, Nash KL, Kool JT (2011) Coral reef recovery dynamics in a changing world. Coral Reefs 30:283–294
Green EP, Bruckner AW (2000) The significance of coral disease, epizootiology for coral reef conservation. Biol Conserv 96:347–361
Harvell D, Altizer S, Cattadori IM, Harrington L, Weil E (2009) Climate change and wildlife diseases: When does the host matter the most? Ecology 90:912–920
Harvell D, Jordán-Dahlgren E, Merkel S, Rosenberg E, Raymundo L, Smith G, Weil E, Willis B (2007) Coral disease, environmental drivers, and the balance between coral and microbial associates. Oceanography 20:172–195
Heron S, Johnston L, Liu G, Geiger E, Maynard J, De La Cour J, Johnson S, Okano R, Benavente D, Burgess T, Iguel J, Perez D, Skirving W, Strong A, Tirak K, Eakin C (2016a) Validation of reef-scale thermal stress satellite products for coral bleaching monitoring. Remote Sens 8:59
Heron SF, Maynard JA, van Hooidonk R, Eakin CM (2016b) Warming trends and bleaching stress of the world’s coral reefs 1985–2012. Sci Rep 6:38402
Heron SF, Willis BL, Skirving WJ, Eakin CM, Page CA, Miller IR (2010) Summer hot snaps and winter conditions: Modelling white syndrome outbreaks on Great Barrier Reef corals. PLoS One 5:e12210
Hill J, Wilkinson C (2004) Methods for ecological monitoring of coral reefs. Australian Institute of Marine Science, Townsville
Hobbs JA, Frisch AJ, Newman SJ, Wakefield CB (2015) Selective impact of disease on coral communities: Outbreak of white syndrome causes significant total mortality of Acropora plate corals. PLoS One 10:e0132528
Hoegh-Guldberg O (1999) Climate change, coral bleaching and the future of the world’s coral reefs. Mar Freshw Res 50:839–866
Holbrook SJ, Adam TC, Edmunds PJ, Schmitt RJ, Carpenter RC, Brooks AJ, Lenihan HS, Briggs CJ (2018) Recruitment drives spatial variation in recovery rates of resilient coral reefs. Sci Rep 8:1–11
Hughes TP, Graham NAJ, Jackson JBC, Mumby PJ, Steneck RS (2010) Rising to the challenge of sustaining coral reef resilience. Trends Ecol Evol 25:633–642
Hughes TP, Barnes ML, Bellwood DR, Cinner JE, Cumming GS, Jackson JBC, Kleypas J, van de Leemput IA, Lough JM, Morrison TH, Palumbi SR, Van Nes EH, Scheffer M (2017a) Coral reefs in the Anthropocene. Nature 546:82–90
Hughes TP, Kerry JT, Álvarez-Noriega M, Álvarez-Romero JG, Anderson KD, Baird AH, Babcock RC, Beger M, Bellwood DR, Berkelmans R, Bridge TC, Butler IR, Byrne M, Cantin NE, Comeau S, Connolly SR, Cumming GS, Dalton SJ, Diaz-Pulido G, Eakin CM, Figueira WF, Gilmour JP, Harrison HB, Heron SF, Hoey AS, Hobbs J-PA, Hoogenboom MO, Kennedy EV, Kuo C, Lough JM, Lowe RJ, Liu G, McCulloch MT, Malcolm HA, McWilliam MJ, Pandolfi JM, Pears RJ, Pratchett MS, Schoepf V, Simpson T, Skirving WJ, Sommer B, Torda G, Wachenfeld DR, Willis BL, Wilson SK (2017b) Global warming and recurrent mass bleaching of corals. Nature 543:373–377
Hughes TP, Anderson KD, Connolly SR, Heron SF, Kerry JT, Lough JM, Baird AH, Baum JK, Berumen ML, Bridge TC, Claar DC, Eakin CM, Gilmour JP, Graham NAJ, Harrison H, Hobbs JPA, Hoey AS, Hoogenboom M, Lowe RJ, McCulloch MT, Pandolfi JM, Pratchett M, Schoepf V, Torda G, Wilson SK (2018a) Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science (80−) 359:80–83
Hughes TP, Kerry JT, Baird AH, Connolly SR, Dietzel A, Eakin CM, Heron SF, Hoey AS, Hoogenboom MO, Liu G, McWilliam MJ, Pears RJ, Pratchett MS, Skirving WJ, Stella JS, Torda G (2018b) Global warming transforms coral reef assemblages. Nature 556:492–496
Hughes TP, Kerry JT, Baird AH, Connolly SR, Chase TJ, Dietzel A, Hill T, Hoey AS, Hoogenboom MO, Jacobson M, Kerswell A, Madin JS, Mieog A, Paley AS, Pratchett MS, Torda G, Woods RM (2019) Global warming impairs stock–recruitment dynamics of corals. Nature 568:387–390
Jones R (2008) Coral bleaching, bleaching-induced mortality, and the adaptive significance of the bleaching response. Mar Biol 154:65–80
Kerry JT, Bellwood DR (2015) Do tabular corals constitute keystone structures for fishes on coral reefs? Coral Reefs 34:41–50
Liu G, Rauenxahn J, Heron S, Eakin C, Skirving WJ, Christensen T, Strong A (2013) NOAA Coral Reef Watch 50 km satellite sea surface temperature-based decision support system for coral bleaching management. 33
Liu G, Heron SF, Eakin CM, Muller-Karger FE, Vega-Rodriguez M, Guild LS, De La Cour JL, Geiger EF, Skirving WJ, Burgess TFR, Strong AE, Harris A, Maturi E, Ignatov A, Sapper J, Li J, Lynds S (2014) Reef-scale thermal stress monitoring of coral ecosystems: new 5-km global products from NOAA Coral Reef Watch. Remote Sens 6:11579–11606
Liu G, Skirving WJ, Geiger EF, De La Cour JL, Marsh BL, Heron SF, Tirak KV, Strong AE, Eakin CM (2017) NOAA coral reef watch’s 5 km satellite coral bleaching heat stress monitoring product suite version 3 and four-month outlook version 4. Reef Encount 32:39–45
MacNeil MA, Mellin C, Matthews S, Wolff NH, McClanahan TR, Devlin M, Drovandi C, Mengersen K, Graham NAJ (2019) Water quality mediates resilience on the Great Barrier Reef. Nat Ecol Evol
Marshall PA, Baird AH (2000) Bleaching of corals on the Great Barrier Reef: Differential susceptibilities among taxa. Coral Reefs 19:155–163
Maynard JA, Anthony KRN, Harvell CD, Burgman MA, Beeden R, Sweatman H, Heron SF, Lamb JB, Willis BL (2011) Predicting outbreaks of a climate-driven coral disease in the Great Barrier Reef. Coral Reefs 30:485–495
Maynard JA, Van Hooidonk R, Eakin CM, Puotinen M, Garren M, Williams G, Heron SF, Lamb J, Weil E, Willis B, Harvell CD (2015) Projections of climate conditions that increase coral susceptibility and pathogen abundance and virulence. Nat Clim Chang 5:688
McClanahan T, Maina J, Moothien-Pillay R, Baker A (2005) Effects of geography, taxa, water flow, and temperature variation on coral bleaching intensity in Mauritius. Mar Ecol Prog Ser 298:131–142
Miller J, Muller E, Rogers C, Waara R, Atkinson A, Whelan KRT, Patterson M, Witcher B (2009) Coral disease following massive bleaching in 2005 causes 60% decline in coral cover on reefs in the US Virgin Islands. Coral Reefs 28:925–937
Muller-Parker G, D’Elia CF, Cook CB (2015) Interactions between corals and their symbiotic algae. Coral Reefs in the Anthropocene. Springer, Netherlands, pp 99–116
Muller EM, Bartels E, Baums IB (2018) Bleaching causes loss of disease resistance within the threatened coral species Acropora cervicornis. Elife 7:1–20
Neal BP, Khen A, Treibitz T, Beijbom O, O’Connor G, Coffroth AM, Knowlton N, Kriegman D, Mitchell BG, Kline DI (2017) Caribbean massive corals not recovering from repeated thermal stress events during 2005–2013. Ecol Evol 7:1339–1353
Page C, Leggat W, Heron S, Choukroun S, Lloyd J, Ainsworth T (2019) Seeking resistance in coral reef ecosystems. The interplay of bio-physical factors and bleaching resistance under a changing climate. BioEssays In print:
Peters EC (2015) Diseases of coral reef organisms. In: Birkeland C (ed) Coral Reefs in the Anthropocene. Springer, Netherlands, Dordrecht, pp 147–178
Pinheiro J, Bates D, DebRoy S, Sarkar D, Team RC (2017) Nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1-128. 2016. R Softw
Precht WF, Gintert BE, Robbart ML, Fura R, Van Woesik R (2016) Unprecedented disease-related coral mortality in southeastern Florida. Sci Rep 6:31374
R Core Team (2017) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Randall CJ, van Woesik R (2015) Contemporary white-band disease in Caribbean corals driven by climate change. Nat Clim Chang 5:375–379
Randall CJ, Jordan-Garza AG, Muller EM, van Woesik R (2014) Relationships between the history of thermal stress and the relative risk of diseases of Caribbean corals. Ecology 95:1981–1994
Roff G, Bejarano S, Bozec YM, Nugues M, Steneck RS, Mumby PJ (2014) Porites and the Phoenix effect: Unprecedented recovery after a mass coral bleaching event at Rangiroa Atoll, French Polynesia. Mar Biol 161:1385–1393
Rogers A, Blanchard JL, Mumby PJ (2014) Vulnerability of coral reef fisheries to a loss of structural complexity. Curr Biol 24:1000–1005
Selig ER, Harvell CD, Bruno JF, Willis BL, Page CA, Casey KS, Sweatman H (2006) Analyzing the relationship between ocean temperature anomalies and coral disease outbreaks at broad spatial scales. Coral reefs Clim Chang Sci Manag 111–128
Shaver EC, Burkepile DE, Silliman BR (2018) Local management actions can increase coral resilience to thermally-induced bleaching. Nat Ecol Evol 2:1075–1079
Sweatman H (2018) Annual summary report on coral reef condition for 2017/18
Sweatman H, Cheal A, Coleman G, Emslie M, Johns K, Jonker M, Miller I, Osborne K (2008) Long-term monitoring of the Great Barrier reef. Status Rep 8:1–379
Willis BL, Page CA, Dinsdale EA (2004) Coral disease on the Great Barrier Reef. In: Rosenberg E, Loya Y (eds) Coral health and disease. Springer, Berlin Heidelberg, pp 69–104
van Hooidonk R, Maynard J, Tamelander J, Jamison G, Ahmadia G, Raymundo L, Williams G, Heron SF, Planes S (2016) Local-scale projections of coral reef futures and implications of the Paris Agreement. Sci Rep 6:e39666
van Oppen MJH, Gates RD, Blackall LL, Cantin N, Chakravarti LJ, Chan WY, Cormick C, Crean A, Damjanovic K, Epstein H, Harrison PL, Jones TA, Miller M, Pears RJ, Peplow LM, Raftos DA, Schaffelke B, Stewart K, Torda G, Wachenfeld D, Weeks AR, Putnam HM (2017) Shifting paradigms in restoration of the world’s coral reefs. Glob Chang Biol 23:3437–3448
Wooldridge SA (2014) Assessing coral health and resilience in a warming ocean: Why looks can be deceptive. Bioessays 36:1041–1049
Work TM, Russell R, Aeby GS (2012) Tissue loss (white syndrome) in the coral Montipora capitata is a dynamic disease with multiple host responses and potential causes. Proc R Soc B Biol Sci 279:4334–4341
Acknowledgements
This study was made possible by financial support from the Great Barrier Reef Marine Park Authority, and funding from the ARC CoE for Coral Reef Studies to B. Willis. Kerryn Bell (Reef Express) is thanked for facilitating the survey trips to Beaver Reef and her infectious passion for corals reefs. Abby Fatland, Allison Paley, Carly Hayk, Carine Lefevre, Kate Quigley, Margaux Hein, Phil Osmond, Saskia McDonald and Svenja Müller are all thanked for their laboratory and fieldwork assistance. SFH was supported by NASA ROSES Ecological Forecasting Grant #16-eco4cast-0032 to the University of Hawaii. The scientific results and conclusions, as well as any views or opinions expressed herein, are those of the author(s) and do not necessarily reflect the views of NOAA or the Department of Commerce. All tagging, surveys and sampling were performed under the auspice of GBRMPA permit G16/38009.1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Topic Editor Morgan S. Pratchett
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Brodnicke, O.B., Bourne, D.G., Heron, S.F. et al. Unravelling the links between heat stress, bleaching and disease: fate of tabular corals following a combined disease and bleaching event. Coral Reefs 38, 591–603 (2019). https://doi.org/10.1007/s00338-019-01813-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00338-019-01813-9