Abstract
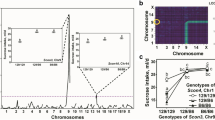
Mice of the C57BL/6ByJ (B6) strain have higher consumption of sucrose, and stronger peripheral neural responses to it, than do mice of the 129P3/J (129) strain. To identify quantitative trait loci (QTLs) responsible for this strain difference and to evaluate the contribution of peripheral taste responsiveness to individual differences in sucrose intake, we produced an intercross (F2) of 627 mice, measured their sucrose consumption in two-bottle choice tests, recorded the electrophysiological activity of the chorda tympani nerve elicited by sucrose in a subset of F2 mice, and genotyped the mice with DNA markers distributed in every mouse chromosome. We confirmed a sucrose consumption QTL (Scon2, or Sac) on mouse chromosome (Chr) 4, harboring the Tas1r3 gene, which encodes the sweet taste receptor subunit TAS1R3 and affects both behavioral and neural responses to sucrose. For sucrose consumption, we also detected five new main-effect QTLs, Scon6 (Chr2), Scon7 (Chr5), Scon8 (Chr8), Scon3 (Chr9), and Scon9 (Chr15), and an epistatically interacting QTL pair Scon4 (Chr1) and Scon3 (Chr9). No additional QTLs for the taste nerve responses to sucrose were detected besides Scon2 (Tas1r3) on Chr4. Identification of the causal genes and variants for these sucrose consumption QTLs may point to novel mechanisms beyond peripheral taste sensitivity that could be harnessed to control obesity and diabetes.







Similar content being viewed by others
Data availability
Available upon request.
Code availability
Not applicable.
Consent for publication
Not applicable.
References
Abbott KN, Morris MJ, Westbrook RF, Reichelt AC (2016) Sex-specific effects of daily exposure to sucrose on spatial memory performance in male and female rats, and implications for estrous cycle stage. Physiol Behav 162:52–60
Ackroff K, Yiin YM, Sclafani A (2010) Post-oral infusion sites that support glucose-conditioned flavor preferences in rats. Physiol Behav 99:402–411
Bachmanov AA (2008) Genetic architecture of sweet taste. In: Weerasinghe DK, DuBois GE (eds) Sweetness and sweeteners: biology, chemistry and psychophysics. American Chemical Society, Washington, DC, pp 18–47
Bachmanov AA, Bosak NP, Floriano WB, Inoue M, Li X, Lin C, Murovets VO, Reed DR, Zolotarev VA, Beauchamp GK (2011) Genetics of sweet taste preferences. Flavour Fragr J 26:286–294
Bachmanov AA, Inoue M, Tordoff MG, Ninomiya Y, Beauchamp GK (1999) Modification of behavioral and neural taste responses to NaCl in C57BL/6 mice: effects of NaCl exposure and DOCA treatment. Physiol Behav 65:817–822
Bachmanov AA, Li X, Reed DR, Ohmen JD, Li S, Chen Z, Tordoff MG, de Jong PJ, Wu C, West DB, Chatterjee A, Ross DA, Beauchamp GK (2001a) Positional cloning of the mouse saccharin preference (Sac) locus. Chem Senses 26:925–933
Bachmanov AA, Reed DR, Li X, Beauchamp GK (2002b) Genetics of sweet taste preferences. Pure Appl Chem 74:1135–1140
Bachmanov AA, Reed DR, Beauchamp GK, Tordoff MG (2002a) Food intake, water intake, and drinking spout side preference of 28 mouse strains. Behav Genet 32:435–443
Bachmanov AA, Reed DR, Ninomiya Y, Inoue M, Tordoff MG, Price RA, Beauchamp GK (1997) Sucrose consumption in mice: major influence of two genetic loci affecting peripheral sensory responses. Mamm Genome 8:545–548
Bachmanov AA, Reed DR, Tordoff MG, Price RA, Beauchamp GK (1996a) Intake of ethanol, sodium chloride, sucrose, citric acid, and quinine hydrochloride solutions by mice: a genetic analysis. Behav Genet 26:563–573
Bachmanov AA, Tordoff MG, Beauchamp GK (1996b) Ethanol consumption and taste preferences in C57BL/6ByJ and 129/J mice. Alcohol Clin Exp Res 20:201–206
Bachmanov AA, Tordoff MG, Beauchamp GK (2001b) Sweetener preference of C57BL/6ByJ and 129P3/J mice. Chem Senses 26:905–913
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B 57:289–300
Blizard DA, Kotlus B, Frank ME (1999) Quantitative trait loci associated with short-term intake of sucrose, saccharin and quinine solutions in laboratory mice. Chem Senses 24:373–385
Blizard DA, McClearn GE (2000) Association between ethanol and sucrose intake in the laboratory mouse: exploration via congenic strains and conditioned taste aversion. Alcohol Clin Exp Res 24:253–258
Broman KW, Wu H, Sen S, Churchill GA (2003) R/qtl: QTL mapping in experimental crosses. Bioinformatics 19:889–890
Bulwa ZB, Sharlin JA, Clark PJ, Bhattacharya TK, Kilby CN, Wang Y, Rhodes JS (2011) Increased consumption of ethanol and sugar water in mice lacking the dopamine D2 long receptor. Alcohol 45:631–639
Choi Y, Chan AP (2015) PROVEAN web server: a tool to predict the functional effect of amino acid substitutions and indels. Bioinformatics 31:2745–2747
Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Varadarajan V, Zou S, Jiang P, Ninomiya Y, Margolskee RF (2003) Detection of sweet and umami taste in the absence of taste receptor T1r3. Science 301:850–853
Danilova V, Hellekant G (2003) Comparison of the responses of the chorda tympani and glossopharyngeal nerves to taste stimuli in C57BL/6J mice. BMC Neurosci 4:5
Davis S, Meltzer PS (2007) GEOquery: a bridge between the Gene Expression Omnibus (GEO) and BioConductor. Bioinformatics 23:1846–1847
de Araujo IE (2012) Multiple reward layers in food reinforcement. In: Neurobiology of sensation and reward. CRC Press, Boca Raton
de Araujo IE, Oliveira-Maia AJ, Sotnikova TD, Gainetdinov RR, Caron MG, Nicolelis MA, Simon SA (2008) Food reward in the absence of taste receptor signaling. Neuron 57:930–941
Dushay JR, Toschi E, Mitten EK, Fisher FM, Herman MA, Maratos-Flier E (2015) Fructose ingestion acutely stimulates circulating FGF21 levels in humans. Mol Metab 4:51–57
Dym CT, Pinhas A, Ginzberg M, Kest B, Bodnar RJ (2007) Genetic variance contributes to naltrexone-induced inhibition of sucrose intake in inbred and outbred mouse strains. Brain Res 1135:136–145
Dym CT, Pinhas A, Robak M, Sclafani A, Bodnar RJ (2009) Genetic variance contributes to dopamine receptor antagonist-induced inhibition of sucrose intake in inbred and outbred mouse strains. Brain Res 1257:40–52
Eny KM, Corey PN, El-Sohemy A (2009) Dopamine D2 receptor genotype (C957T) and habitual consumption of sugars in a free-living population of men and women. J Nutrigenet Nutrigenomics 2:235–242
Hajnal A, Covasa M, Bello NT (2005) Altered taste sensitivity in obese, prediabetic OLETF rats lacking CCK-1 receptors. Am J Physiol Regul Integr Comp Physiol 289:R1675–R1686
Han W, Tellez LA, Niu J, Medina S, Ferreira TL, Zhang X, Su J, Tong J, Schwartz GJ, van den Pol A, de Araujo IE (2016) Striatal dopamine links gastrointestinal rerouting to altered sweet appetite. Cell Metab 23:103–112
Hofmann WE, Liu X, Bearden CM, Harper ME, Kozak LP (2001) Effects of genetic background on thermoregulation and fatty acid-induced uncoupling of mitochondria in UCP1-deficient mice. J Biol Chem 276:12460–12465
Hwang LD, Lin C, Gharahkhani P, Cuellar-Partida G, Ong JS, An J, Gordon SD, Zhu G, MacGregor S, Lawlor DA, Breslin PAS, Wright MJ, Martin NG, Reed DR (2019) New insight into human sweet taste: a genome-wide association study of the perception and intake of sweet substances. Am J Clin Nutr 109(6):724–1737
Inoue M, Beauchamp GK, Bachmanov AA (2004a) Gustatory neural responses to umami taste stimuli in C57BL/6ByJ and 129P3/J mice. Chem Senses 29:789–795
Inoue M, Glendinning JI, Theodorides ML, Harkness S, Li X, Bosak N, Beauchamp GK, Bachmanov AA (2007) Allelic variation of the Tas1r3 taste receptor gene selectively affects taste responses to sweeteners: evidence from 129.B6-Tas1r3 congenic mice. Physiol Genomics 32:82–94
Inoue M, Li X, McCaughey SA, Beauchamp GK, Bachmanov AA (2001a) Soa genotype selectively affects mouse gustatory neural responses to sucrose octaacetate. Physiol Genomics 5:181–186
Inoue M, McCaughey SA, Bachmanov AA, Beauchamp GK (2001b) Whole-nerve chorda tympani responses to sweeteners in C57BL/6ByJ and 129P3/J mice. Chem Senses 26:915–923
Inoue M, Reed DR, Li X, Tordoff MG, Beauchamp GK, Bachmanov AA (2004b) Allelic variation of the Tas1r3 taste receptor gene selectively affects behavioral and neural taste responses to sweeteners in the F2 hybrids between C57BL/6ByJ and 129P3/J mice. J Neurosci 24:2296–2303
Inoue M, Tordoff MG (1998) Calcium deficiency alters chorda tympani nerve responses to oral calcium chloride. Physiol Behav 63:297–303
Jerez-Timaure NC, Kearney F, Simpson EB, Eisen EJ, Pomp D (2004) Characterization of QTL with major effects on fatness and growth on mouse chromosome 2. Obes Res 12:1408–1420
Kawai K, Sugimoto K, Nakashima K, Miura H, Ninomiya Y (2000) Leptin as a modulator of sweet taste sensitivities in mice. Proc Natl Acad Sci USA 97:11044–11049
Keane TM, Goodstadt L, Danecek P, White MA, Wong K, Yalcin B, Heger A, Agam A, Slater G, Goodson M, Furlotte NA, Eskin E, Nellaker C, Whitley H, Cleak J, Janowitz D, Hernandez-Pliego P, Edwards A, Belgard TG, Oliver PL, McIntyre RE, Bhomra A, Nicod J, Gan X, Yuan W, van der Weyden L, Steward CA, Bala S, Stalker J, Mott R, Durbin R, Jackson IJ, Czechanski A, Guerra-Assuncao JA, Donahue LR, Reinholdt LG, Payseur BA, Ponting CP, Birney E, Flint J, Adams DJ (2011) Mouse genomic variation and its effect on phenotypes and gene regulation. Nature 477:289–294
Khaled ML, Bykhovskaya Y, Gu C, Liu A, Drewry MD, Chen Z, Mysona BA, Parker E, McNabb RP, Yu H, Lu X, Wang J, Li X, Al-Muammar A, Rotter JI, Porter LF, Estes A, Watsky MA, Smith SB, Xu H, Abu-Amero KK, Kuo A, Shears SB, Rabinowitz YS, Liu Y (2019) PPIP5K2 and PCSK1 are candidate genetic contributors to familial keratoconus. Sci Rep 9:19406
Korostynski M, Piechota M, Kaminska D, Solecki W, Przewlocki R (2007) Morphine effects on striatal transcriptome in mice. Genome Biol 8:R128
Lemon CH, Margolskee RF (2009) Contribution of the T1r3 taste receptor to the response properties of central gustatory neurons. J Neurophysiol 101:2459–2471
Li X, Inoue M, Reed DR, Huque T, Puchalski RB, Tordoff MG, Ninomiya Y, Beauchamp GK, Bachmanov AA (2001) High-resolution genetic mapping of the saccharin preference locus (Sac) and the putative sweet taste receptor (T1R1) gene (Gpr70) to mouse distal Chromosome 4. Mamm Genome 12:13–16
Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E (2002) Human receptors for sweet and umami taste. Proc Natl Acad Sci USA 99:4692–4696
Lin C, Inoue M, Ishiwatari Y, Bosak NP, Li X, Reed DR, Beauchamp GK, Bachmanov AA (2015) Genome-wide analysis of quantitative trait loci for behavioral and neural taste responses to sweeteners in F2 hybrids between C57BL/6ByJ and 129P3/J Mice (Abstract). Chem Senses 40:617–618
Lin C, Theodorides ML, McDaniel AH, Tordoff MG, Zhang Q, Li X, Bosak N, Bachmanov AA, Reed DR (2013) QTL analysis of dietary obesity in C57BL/6byj X 129P3/J F2 mice: diet- and sex-dependent effects. PLoS ONE 8:e68776
Lin C, Tordoff MG, Li X, Bosak NP, Inoue M, Ishiwatari Y, Chen L, Beauchamp GK, Bachmanov AA, Reed DR (2021) Genetic controls of mouse Tas1r3-independent sucrose intake. Mamm Genome. https://doi.org/10.1007/s00335-021-09860-w
Lionikas A, Blizard DA, Vandenbergh DJ, Glover MG, Stout JT, Vogler GP, McClearn GE, Larsson L (2003) Genetic architecture of fast- and slow-twitch skeletal muscle weight in 200-day-old mice of the C57BL/6J and DBA/2J lineage. Physiol Genomics 16:141–152
Lush IE (1989) The genetics of tasting in mice. VI. Saccharin, acesulfame, dulcin and sucrose. Genet Res 53:95–99
Marks-Kaufman R, Hamm MW, Barbato GF (1989) The effects of dietary sucrose on opiate receptor binding in genetically obese (ob/ob) and lean mice. J Am Coll Nutr 8:9–14
May CE, Vaziri A, Lin YQ, Grushko O, Khabiri M, Wang QP, Holme KJ, Pletcher SD, Freddolino PL, Neely GG, Dus M (2019) High dietary sugar reshapes sweet taste to promote feeding behavior in Drosophila melanogaster. Cell Rep 27:1675-1685.e1677
McCaughey SA (2007) Taste-evoked responses to sweeteners in the nucleus of the solitary tract differ between C57BL/6ByJ and 129P3/J mice. J Neurosci 27:35–45
McCaughey SA, Glendinning JI (2013) Experience with sugar modifies behavioral but not taste-evoked medullary responses to sweeteners in mice. Chem Senses 38:793–802
McLaughlin SK, McKinnon PJ, Margolskee RF (1992) Gustducin is a taste-cell-specific G protein closely related to the transducins. Nature 357:563–569
Melo JA, Shendure J, Pociask K, Silver LM (1996) Identification of sex-specific quantitative trait loci controlling alcohol preference in C57BL/6 mice. Nat Genet 13:147–153
Mi H, Muruganujan A, Thomas PD (2013) PANTHER in 2013: modeling the evolution of gene function, and other gene attributes, in the context of phylogenetic trees. Nucleic Acids Res 41:D377–D386
Miyasaka A, Imoto T (1995) Electrophysiological characterization of the inhibitory effect of a novel peptide gurmarin on the sweet taste response in rats. Brain Res 676:63–68
Nakamura Y, Sanematsu K, Ohta R, Shirosaki S, Koyano K, Nonaka K, Shigemura N, Ninomiya Y (2008) Diurnal variation of human sweet taste recognition thresholds is correlated with plasma leptin levels. Diabetes 57:2661–2665
Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJ, Zuker CS (2002) An amino-acid taste receptor. Nature 416:199–202
Nelson G, Hoon MA, Chandrashekar J, Zhang Y, Ryba NJ, Zuker CS (2001) Mammalian sweet taste receptors. Cell 106:381–390
Ninomiya Y, Bachmanov AA, Yatabe A, Beauchamp GK (1998) NaCl-preferring NZB/BlNJ mice and NaCl-avoiding CBA/J mice have similar amiloride inhibition of chorda tympani responses to NaCl. Chem Senses 23:411–415
Ninomiya Y, Funakoshi M (1989) Peripheral neural basis for behavioural discrimination between glutamate and the four basic taste substances in mice. Comp Biochem Physiol 92:371–376
Ninomiya Y, Kajiura H, Mochizuki K (1993) Differential taste responses of mouse chorda tympani and glossopharyngeal nerves to sugars and amino acids. Neurosci Lett 163:197–200
Nuzhdin SV, Pasyukova EG, Dilda CL, Zeng ZB, Mackay TF (1997) Sex-specific quantitative trait loci affecting longevity in Drosophila melanogaster. Proc Natl Acad Sci USA 94:9734–9739
Olszewski PK, Levine AS (2007) Central opioids and consumption of sweet tastants: when reward outweighs homeostasis. Physiol Behav 91:506–512
Peirce JL, Derr R, Shendure J, Kolata T, Silver LM (1998) A major influence of sex-specific loci on alcohol preference in C57Bl/6 and DBA/2 inbred mice. Mamm Genome 9:942–948
Perez CA, Huang L, Rong M, Kozak JA, Preuss AK, Zhang H, Max M, Margolskee RF (2002) A transient receptor potential channel expressed in taste receptor cells. Nat Neurosci 5:1169–1176
Qin Y, Sukumaran SK, Jyotaki M, Redding K, Jiang P, Margolskee RF (2018) Gli3 is a negative regulator of Tas1r3-expressing taste cells. PLoS Genet 14:e1007058
Quiroga AD, Li L, Trotzmuller M, Nelson R, Proctor SD, Kofeler H, Lehner R (2012) Deficiency of carboxylesterase 1/esterase-x results in obesity, hepatic steatosis, and hyperlipidemia. Hepatology 56:2188–2198
Raymond E, Soccio ZL, Chen ER, Foong YH, Benson KK, Dispirito JR, Mullican SE, Emmett MJ, Briggs ER, Peed LC, Dzeng RK, Medina CJ, Jolivert JF, Kissig M, Rajapurkar SR, Damle M, Lim H-W, Won K-J, Seale P, Steger DJ, Lazar MA (2017) Targeting PPARγ in the epigenome rescues genetic metabolic defects in mice. J Clin Investig 127(4):1451–1462
Reed DR, Bachmanov AA, Beauchamp GK, Tordoff MG, Price RA (1997) Heritable variation in food preferences and their contribution to obesity. Behav Genet 27:373–387
Reed DR, Li S, Li X, Huang L, Tordoff MG, Starling-Roney R, Taniguchi K, West DB, Ohmen JD, Beauchamp GK, Bachmanov AA (2004) Polymorphisms in the taste receptor gene (Tas1r3) region are associated with saccharin preference in 30 mouse strains. J Neurosci 24:938–946
Robinson MD, McCarthy DJ, Smyth GK (2010) edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26:139–140
Ruff JS, Suchy AK, Hugentobler SA, Sosa MM, Schwartz BL, Morrison LC, Gieng SH, Shigenaga MK, Potts WK (2013) Human-relevant levels of added sugar consumption increase female mortality and lower male fitness in mice. Nat Commun 4:2245
Schneider LH (1989) Orosensory self-stimulation by sucrose involves brain dopaminergic mechanisms. Ann NY Acad Sci 575:307–319
Sclafani A (2001) Post-ingestive positive controls of ingestive behavior. Appetite 36:79–83
Sclafani A (2004) Oral and postoral determinants of food reward. Physiol Behav 81:773–779
Sclafani A (2006a) Sucrose motivation in sweet “sensitive” (C57BL/6J) and “subsensitive” (129P3/J) mice measured by progressive ratio licking. Physiol Behav 87:734–744
Sclafani A (2006b) Oral, post-oral and genetic interactions in sweet appetite. Physiol Behav 89:525–530
Sclafani A (2006c) Enhanced sucrose and Polycose preference in sweet “sensitive” (C57BL/6J) and “subsensitive” (129P3/J) mice after experience with these saccharides. Physiol Behav 87:745–756
Sclafani A (2007) Fat and sugar flavor preference and acceptance in C57BL/6J and 129 mice: experience attenuates strain differences. Physiol Behav 90:602–611
Sclafani A, Ackroff K (2004) The relationship between food reward and satiation revisited. Physiol Behav 82:89–95
Sclafani A, Glendinning JI (2005) Sugar and fat conditioned flavor preferences in C57BL/6J and 129 mice: oral and postoral interactions. Am J Physiol Regul Integr Comp Physiol 289:R712–R720
Sclafani A, Koepsell H, Ackroff K (2016) SGLT1 sugar transporter/sensor is required for post-oral glucose appetition. Am J Physiol Regul Integr Comp Physiol 310:R631–R639
Sedelis M, Hofele K, Schwarting RK, Huston JP, Belknap JK (2003) Chromosomal loci influencing the susceptibility to the parkinsonian neurotoxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. J Neurosci 23:8247–8253
Shin YK, Martin B, Golden E, Dotson CD, Maudsley S, Kim W, Jang HJ, Mattson MP, Drucker DJ, Egan JM, Munger SD (2008) Modulation of taste sensitivity by GLP-1 signaling. J Neurochem 106:455–463
Shingai T, Beidler LM (1985) Response characteristics of three taste nerves in mice. Brain Res 335:245–249
Sinclair MS, Perea-Martinez I, Dvoryanchikov G, Yoshida M, Nishimori K, Roper SD, Chaudhari N (2010) Oxytocin signaling in mouse taste buds. PLoS ONE 5:e11980
Smyth GK (2005) Limma: linear models for microarray data. In: Gentleman R, Carey V, Dudoit S, Irizarry R, Huber W (eds) Bioinformatics and computational biology solutions using R and Bioconductor. Springer, New York, pp 397–420
Soberg S, Sandholt CH, Jespersen NZ, Toft U, Madsen AL, von Holstein-Rathlou S, Grevengoed TJ, Christensen KB, Bredie WLP, Potthoff MJ, Solomon TPJ, Scheele C, Linneberg A, Jorgensen T, Pedersen O, Hansen T, Gillum MP, Grarup N (2017) FGF21 is a sugar-induced hormone associated with sweet intake and preference in humans. Cell Metab 25:1045-1053.e1046
Solberg LC, Baum AE, Ahmadiyeh N, Shimomura K, Li R, Turek FW, Churchill GA, Takahashi JS, Redei EE (2004) Sex- and lineage-specific inheritance of depression-like behavior in the rat. Mamm Genome 15:648–662
Suliman HB, Ali M, Piantadosi CA (2004) Superoxide dismutase-3 promotes full expression of the EPO response to hypoxia. Blood 104:43–50
Thanarajah SE, Backes H, DiFeliceantonio AG, Albus K, Cremer AL, Hanssen R, Lippert RN, Cornely OA, Small DM, Bruning JC, Tittgemeyer M (2019) Food intake recruits orosensory and post-ingestive dopaminergic circuits to affect eating desire in humans. Cell Metab 29:695-706.e694
Tordoff MG, Bachmanov AA (2001) Monell mouse taste phenotyping project. Monell Chemical Senses Center. www.monell.org/MMTPP. Accessed 1 Mar 2021
Tordoff MG, Bachmanov AA (2002) Influence of test duration on the sensitivity of the two-bottle choice test. Chem Senses 27:759–768
Trayhurn P, Bing C (2006) Appetite and energy balance signals from adipocytes. Philos Trans R Soc Lond B 361:1237–1249
von Holstein-Rathlou S, BonDurant LD, Peltekian L, Naber MC, Yin TC, Claflin KE, Urizar AI, Madsen AN, Ratner C, Holst B, Karstoft K, Vandenbeuch A, Anderson CB, Cassell MD, Thompson AP, Solomon TP, Rahmouni K, Kinnamon SC, Pieper AA, Gillum MP, Potthoff MJ (2016) FGF21 mediates endocrine control of simple sugar intake and sweet taste preference by the liver. Cell Metab 23:335–343
Wellcome Sanger Institute (2011) Mouse Genomes Project—Query SNPs, indels or SVs. https://www.sanger.ac.uk/sanger/Mouse_SnpViewer/rel-1505. Accessed 1 Mar 2021
Yalcin B, Wong K, Agam A, Goodson M, Keane TM, Gan X, Nellaker C, Goodstadt L, Nicod J, Bhomra A, Hernandez-Pliego P, Whitley H, Cleak J, Dutton R, Janowitz D, Mott R, Adams DJ, Flint J (2011) Sequence-based characterization of structural variation in the mouse genome. Nature 477:326–329
Yang H, Wang JR, Didion JP, Buus RJ, Bell TA, Welsh CE, Bonhomme F, Yu AH, Nachman MW, Pialek J, Tucker P, Boursot P, McMillan L, Churchill GA, de Villena FP (2011) Subspecific origin and haplotype diversity in the laboratory mouse. Nat Genet 43:648–655
Yee KK, Sukumaran SK, Kotha R, Gilbertson TA, Margolskee RF (2011) Glucose transporters and ATP-gated K+ (KATP) metabolic sensors are present in type 1 taste receptor 3 (T1r3)-expressing taste cells. Proc Natl Acad Sci USA 108:5431–5436
Yirmiya R, Lieblich I, Liebeskind JC (1988) Reduced saccharin preference in CXBK (opioid receptor-deficient) mice. Brain Res 438:339–342
Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJ, Zuker CS (2003) The receptors for mammalian sweet and umami taste. Cell 115:255–266
Acknowledgements
We gratefully acknowledge Maria L. Theodorides, Zakiyyah Smith, Mauricio Avigdor, and Amy Colihan for assistance with animal breeding. We also acknowledge Richard Copeland and the consistent high-quality assistance of the Animal Care Staff at the Monell Chemical Senses Center and thank them for their service.
Funding
National Institutes of Health Grants R01 DC00882 (AAB and GKB), R01 AA11028 (AAB and MGT), R03 DC03854, R03 TW007429 (AAB), R03 DC03509, R01 DC04188, R01 DK55853, R01 DK094759, R01 DK058797, P30 DC011735, S10 OD018125, S10 RR025607, S10 RR026752, and G20 OD020296 (DRR) and Institutional Funds from the Monell Chemical Senses Center supported this work, including genotyping and the purchase of equipment. The Center for Inherited Disease Research through the auspices of the National Institutes of Health provided genotyping services.
Author information
Authors and Affiliations
Contributions
AAB, DRR, MGT, and GKB designed the study. AAB bred and phenotyped mice. MI conducted electrophysiological experiments. CL, XL, NPB, and YI genotyped mice. CL, YI, AAB, and DRR analyzed data. CL and DRR wrote the paper. AAB, MGT, and GKB commented and edited the paper. All authors read the paper and approved its contents.
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there are no conflicts of interest.
Ethical approval
All animal procedures in this study were approved by the Institutional Animal Care and Use Committee of the Monell Chemical Senses Center.
Informed consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Accession IDs: MGI:2661414 (Scon2), MGI:3783324 (Scon3), MGI:5489743 (Scon4), MGI:6451653 (Scon6), MGI:6451654 (Scon7), MGI:6451655 (Scon8), MGI:6451656 (Scon9).
Supplementary Information
Rights and permissions
About this article
Cite this article
Lin, C., Inoue, M., Li, X. et al. Genetics of mouse behavioral and peripheral neural responses to sucrose. Mamm Genome 32, 51–69 (2021). https://doi.org/10.1007/s00335-021-09858-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00335-021-09858-4