Abstract
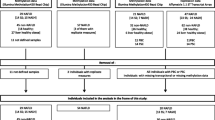
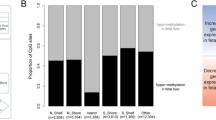
Polymorphisms and decreased activity of methylenetetrahydrofolate reductase (MTHFR) are linked to disease, including cancer. However, epigenetic regulation has not been thoroughly studied. Our goal was to generate DNA methylation profiles of murine/human MTHFR gene regions and examine methylation in brain and liver tumors. Pyrosequencing in four murine tissues revealed minimal DNA methylation in the CpG island. Higher methylation was seen in liver or intestine in the CpG island shore 5′ to the upstream translational start site or in another region 3′ to the downstream start site. In the latter region, there was negative correlation between expression and methylation. Three orthologous regions were investigated in human MTHFR, as well as a fourth region between the two translation start sites. We found significantly increased methylation in three regions (not the CpG island) in pediatric astrocytomas compared with control brain, with decreased expression in tumors. Methylation in hepatic carcinomas was also increased in the three regions compared with normal liver, but the difference was significant for only one CpG. This work, the first overview of the Mthfr/MTHFR epigenetic landscape, suggests regulation through methylation in some regions, demonstrates increased methylation/decreased expression in pediatric astrocytomas, and should serve as a resource for future epigenetic studies.








Similar content being viewed by others
References
Antequera F et al (1990) High levels of de novo methylation and altered chromatin structure at CpG islands in cell lines. Cell 62:503–514
Bailey LB, Caudill MA (2012) Folate. In: Erdman JW, MacDonald IA, Zeisel SH (eds) Present knowledge in nutrition. Oxford, Wiley-Blackwell, pp 321–342
Botto LD, Yang Q (2000) 5,10-Methylenetetrahydrofolate reductase gene variants and congenital anomalies: a HuGE review. Am J Epidemiol 151:862–877
Chen Z et al (2001) Mice deficient in methylenetetrahydrofolate reductase exhibit hyperhomocysteinemia and decreased methylation capacity, with neuropathology and aortic lipid deposition. Hum Mol Genet 10:433–443
Christensen KE, Rozen R (2010) Genetic variation: effect on folate metabolism and health. In: Bailey LB (ed) Folate in health and disease. CRC Press, Boca Raton, pp 75–131
Christensen KE et al (2010) Steatosis in mice is associated with gender, folate intake, and expression of genes of one-carbon metabolism. J Nutr 140:1736–1741
Christensen KE et al (2015) High folic acid consumption leads to pseudo-MTHFR deficiency, altered lipid metabolism, and liver injury in mice. Am J Clin Nutr 101:646–658
Devlin AM et al (2004) Effect of Mthfr genotype on diet-induced hyperhomocysteinemia and vascular function in mice. Blood 103:2624–2629
Esteller M (2007) Cancer epigenomics: DNA methylomes and histone-modification maps. Nat Rev Genet 8:286–298
Feinberg AP, Tycko B (2004) The history of cancer epigenetics. Nat Rev Cancer 4:143–153
Fontebasso AM et al (2014) Recurrent somatic mutations in ACVR1 in pediatric midline high-grade astrocytoma. Nat Genet 46:462–466
Frosst P et al (1995) A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet 10:111–113
Gilbody S et al (2007) Methylenetetrahydrofolate reductase (MTHFR) genetic polymorphisms and psychiatric disorders: a HuGE review. Am J Epidemiol 165:1–13
Giovannucci E et al (1995) Alcohol, low-methionine–low-folate diets, and risk of colon cancer in men. J Natl Cancer Inst 87:265–273
Goyette P et al (1994) Human methylenetetrahydrofolate reductase: isolation of cDNA mapping and mutation identification. Nat Genet 7:195–200
Goyette P et al (1998) Gene structure of human and mouse methylenetetrahydrofolate reductase (MTHFR). Mamm Genome 9:652–656
Hansen RS, Gartler SM (1990) 5-Azacytidine-induced reactivation of the human × chromosome-linked PGK1 gene is associated with a large region of cytosine demethylation in the 5′ CpG island. Proc Natl Acad Sci USA 87:4174–4178
Heijmans BT et al (2003) A common variant of the methylenetetrahydrofolate reductase gene (1p36) is associated with an increased risk of cancer. Cancer Res 63:1249–1253
Irizarry RA et al (2009) Genome-wide methylation analysis of human colon cancer reveals similar hypo- and hypermethylation at conserved tissue-specific CpG island shores. Nat Genet 41:178–186
Jones PA, Baylin SB (2002) The fundamental role of epigenetic events in cancer. Nat Rev Genet 3:415–428
Junker R et al (2001) Infant methylenetetrahydrofolate reductase 677TT genotype is a risk factor for congenital heart disease. Cardiovasc Res 51:251–254
Khazamipour N et al (2009) MTHFR promoter hypermethylation in testicular biopsies of patients with non-obstructive azoospermia: the role of epigenetics in male infertility. Hum Reprod 24:2361–2364
Kleinman CL et al (2014) Fusion of TTYH1 with the C19MC microRNA cluster drives expression of a brain-specific DNMT3B isoform in the embryonal brain tumor ETMR. Nat Genet 46:39–44
Knock E et al (2006) Low dietary folate initiates intestinal tumors in mice, with altered expression of G2-M checkpoint regulators polo-like kinase 1 and cell division cycle 25c. Cancer Res 66:10349–10356
Knock E et al (2008) Strain differences in mice highlight the role of DNA damage in neoplasia induced by low dietary folate. J Nutr 138:653–658
Knock E et al (2011) Susceptibility to intestinal tumorigenesis in folate-deficient mice may be influenced by variation in one-carbon metabolism and DNA repair. J Nutr Biochem 22:1022–1029
Kulis M et al (2013) Intragenic DNA methylation in transcriptional regulation, normal differentiation and cancer. Biochim Biophys Acta 1829:1161–1174
Laird PW (2003) The power and the promise of DNA methylation markers. Nat Rev Cancer 3:253–266
Landan G et al (2012) Epigenetic polymorphism and the stochastic formation of differentially methylated regions in normal and cancerous tissues. Nat Genet 44:1207–1214
Lawrance AK et al (2009) Methylenetetrahydrofolate reductase deficiency and low dietary folate reduce tumorigenesis in Apc min/+ mice. Gut 58:805–811
Leclerc D et al (2003) Characterization of a pseudogene for murine methylenetetrahydrofolate reductase. Mol Cell Biochem 252:391–395
Leclerc D et al (2013) Differential gene expression and methylation in the retinoid/PPARA pathway and of tumor suppressors may modify intestinal tumorigenesis induced by low folate in mice. Mol Nutr Food Res 57:686–697
Lev Maor G et al (2015) The alternative role of DNA methylation in splicing regulation. Trends Genet 31:274–280
Li D, Rozen R (2006) Maternal folate deficiency affects proliferation, but not apoptosis, in embryonic mouse heart. J Nutr 136:1774–1778
Liew SC, Gupta ED (2015) Methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism: epidemiology, metabolism and the associated diseases. Eur J Med Genet 58:1–10
Ma J et al (1997) Methylenetetrahydrofolate reductase polymorphism, dietary interactions, and risk of colorectal cancer. Cancer Res 57:1098–1102
Maunakea AK et al (2010) Conserved role of intragenic DNA methylation in regulating alternative promoters. Nature 466:253–257
Noushmehr H et al (2010) Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell 17:510–522
Pickell L et al (2011) Targeted insertion of two Mthfr promoters in mice reveals temporal- and tissue-specific regulation. Mamm Genome 22:635–647
Pike BL et al (2008) DNA methylation profiles in diffuse large B-cell lymphoma and their relationship to gene expression status. Leukemia 22:1035–1043
Powell SM et al (1992) APC mutations occur early during colorectal tumorigenesis. Nature 359:235–237
Rotondo JC et al (2012) Methylenetetrahydrofolate reductase gene promoter hypermethylation in semen samples of infertile couples correlates with recurrent spontaneous abortion. Hum Reprod 27:3632–3638
Sibani S et al (2002) Studies of methionine cycle intermediates (SAM, SAH), DNA methylation and the impact of folate deficiency on tumor numbers in Min mice. Carcinogenesis 23:61–65
Stankova J et al (2005) Antisense inhibition of methylenetetrahydrofolate reductase reduces cancer cell survival in vitro and tumor growth in vivo. Clin Cancer Res 11:2047–2052
Sturm D et al (2012) Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell 22:425–437
Tran P et al (2002) Multiple transcription start sites and alternative splicing in the methylenetetrahydrofolate reductase gene result in two enzyme isoforms. Mamm Genome 13:483–492
Vaissiere T et al (2009) Quantitative analysis of DNA methylation profiles in lung cancer identifies aberrant DNA methylation of specific genes and its association with gender and cancer risk factors. Cancer Res 69:243–252
van der Put NM et al (1995) Mutated methylenetetrahydrofolate reductase as a risk factor for spina bifida. Lancet 346:1070–1071
Vandesompele J et al (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3:1–11
Wu W et al (2010) Idiopathic male infertility is strongly associated with aberrant promoter methylation of methylenetetrahydrofolate reductase (MTHFR). PLoS ONE 5:e13884
Acknowledgments
This work was supported by the Canadian Institutes of Health Research (Grant No. MOP 43232 to RR). NL was supported by a Fellowship from the Fonds de Recherche du Québec - Santé and the RI-MUHC Desjardins Fellowship. The Research Institute is supported by a Centres Grant from the Fonds de Recherche du Québec - Santé.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
The authors declare that the experiments comply with the current laws of the country in which they were performed.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lévesque, N., Leclerc, D., Gayden, T. et al. Murine diet/tissue and human brain tumorigenesis alter Mthfr/MTHFR 5′-end methylation. Mamm Genome 27, 122–134 (2016). https://doi.org/10.1007/s00335-016-9624-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00335-016-9624-0