Abstracts
Objectives
Subjective cognitive decline (SCD) may be a preclinical stage of Alzheimer’s disease (AD). Neuroimaging studies suggest that abnormal brain connectivity plays an important role in the pathophysiology of SCD. However, most previous studies focused on single modalities only. Multimodal combinations can more effectively utilize various information and little is known about their diagnostic value in SCD.
Methods
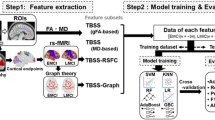
One hundred ten SCD individuals and well-matched healthy controls (HCs) were recruited in this study (the primary sample: 35 SCD and 36 HC; the validation sample: 21 SCD and 18 HC). Multimodal imaging data were used to construct functional, anatomical, and morphological networks, respectively. These networks were used in combination with a multiple kernel learning-support vector machine to predict SCD individuals. We validated our model on another independent sample. Multiple linear regression (MLR) analyses were conducted to investigate the relationships among network metrics, cognition, and pathological biomarkers.
Results
We found that the characteristics identified from the multimodal network were primarily located in the default mode network (DMN) and salience network (SN), achieving an accuracy of 88.73% (an accuracy of 79.49% for an independent sample) based on the integration of the three modalities. MLR analyses showed that increased AV45 SUVRs were significantly associated with impaired memory function, the enhanced functional connectivity, and the decreased morphological connectivity.
Conclusion
This study suggests that abnormal multimodal connections within DMN and SN can be used as effective biomarkers to identify SCD and provide insight into understanding the pathophysiological mechanisms underlying SCD.
Key Points
• Multimodal brain networks improve the detection accuracy of SCD.
• Abnormal connections within DMN and SN can be used as effective biomarkers for the identification of SCD.



Similar content being viewed by others
Abbreviations
- Aβ:
-
Amyloid-β
- AD:
-
Alzheimer’s disease
- ADNI:
-
Alzheimer’s Disease Neuroimaging Initiative
- APOE:
-
Apolipoprotein E
- CAT:
-
Computational Anatomy Toolbox
- CCI:
-
Cognitive Change Index
- CDR:
-
Clinical dementia rating
- CDT:
-
Clock-Drawing Test
- CEN:
-
Central executive network
- CI:
-
Confidence intervals
- CSF:
-
Cerebrospinal fluid
- DELCODE:
-
DZNE-Longitudinal Cognitive Impairment and Dementia
- DMN:
-
Default mode network
- DPABI:
-
Data Processing & Analysis for Brain Imaging
- DTI:
-
Diffusion tensor imaging
- DWI:
-
Diffusion-weighted imaging
- FA:
-
Fractional anisotropy
- FDR:
-
False discovery rate
- fMRI:
-
Resting-state functional magnetic resonance imaging
- GDS:
-
Geriatric depression scale
- GM:
-
Gray matter
- HC:
-
Healthy controls
- JSS:
-
Jensen-Shannon distance-based similarity
- LOOCV:
-
Leave-one-out cross-validation
- MCI:
-
Mild cognitive impairment
- MKL-SVM:
-
Multiple kernel learning SVM
- MMSE:
-
Mini-Mental State Examination
- MNI:
-
Montreal Neurological Institute
- PANDA:
-
Pipeline for Analyzing braiN Diffusion imAges
- PET:
-
Positron emission tomography
- RAVLT:
-
Rey Auditory Verbal Learning Test
- ROC:
-
Receiver operating characteristic curve
- ROIs:
-
Regions of interest
- SCD:
-
Subjective cognitive decline
- SD:
-
Standard deviation
- sMRI:
-
Structural MRI
- SN:
-
Salience network
- SPM:
-
Statistical parametric mapping
- SUVRs:
-
Standard uptake value ratios
- SVM:
-
Support vector machine
- TE:
-
Echo time
- TI:
-
Inversion time
- TMT:
-
Trail-Making Test
- TR:
-
Repetition time
- WM:
-
White matter
- WMS-LM:
-
Wechsler Memory Scale-Logical Memory
References
GBD 2016 Dementia Collaborators (2019) Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 18:88–106
Busche MA, Konnerth A (2016) Impairments of neural circuit function in Alzheimer’s disease. Philos Trans R Soc Lond B Biol Sci 371:20150429
Jessen F, Amariglio RE, van Boxtel M et al (2014) A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement 10:844–852
Snitz BE, Wang T, Cloonan YK et al (2018) Risk of progression from subjective cognitive decline to mild cognitive impairment: the role of study setting. Alzheimers Dement 14:734–742
Molinuevo JL, Rabin LA, Amariglio R et al (2017) Implementation of subjective cognitive decline criteria in research studies. Alzheimers Dement 13:296–311
Viviano RP, Damoiseaux JS (2020) Functional neuroimaging in subjective cognitive decline: current status and a research path forward. Alzheimers Res Ther 12:23
Chiesa PA, Cavedo E, Grothe MJ et al (2019) Relationship between basal forebrain resting-state functional connectivity and brain amyloid-β deposition in cognitively intact older adults with subjective memory complaints. Radiology 290:167–176
Shu N, Wang X, Bi Q, Zhao T, Han Y (2018) Disrupted topologic efficiency of white matter structural connectome in individuals with subjective cognitive decline. Radiology 286:229–238
Verfaillie SCJ, Slot RER, Dicks E et al (2018) A more randomly organized grey matter network is associated with deteriorating language and global cognition in individuals with subjective cognitive decline. Hum Brain Mapp 39:3143–3151
Mutlu J, Landeau B, Gaubert M, de La Sayette V, Desgranges B, Chételat G (2017) Distinct influence of specific versus global connectivity on the different Alzheimer’s disease biomarkers. Brain 140:3317–3328
Sun Y, Yin Q, Fang R et al (2014) Disrupted functional brain connectivity and its association to structural connectivity in amnestic mild cognitive impairment and Alzheimer’s disease. PLoS One 9:e96505
Rathore S, Habes M, Iftikhar MA, Shacklett A, Davatzikos C (2017) A review on neuroimaging-based classification studies and associated feature extraction methods for Alzheimer’s disease and its prodromal stages. Neuroimage 155:530–548
Bryan RN (2016) Machine learning applied to Alzheimer disease. Radiology 281:665–668
Peter J, Scheef L, Abdulkadir A et al (2014) Gray matter atrophy pattern in elderly with subjective memory impairment. Alzheimers Dement 10:99–108
Yang L, Yan Y, Wang Y et al (2018) Gradual disturbances of the amplitude of low-frequency fluctuations (ALFF) and fractional ALFF in Alzheimer spectrum. Front Neurosci 12:975
Chételat G (2018) Multimodal neuroimaging in Alzheimer’s disease: early diagnosis, physiopathological mechanisms, and impact of lifestyle. J Alzheimers Dis 64:S199–S211
Risacher SL, Kim S, Nho K et al (2015) APOE effect on Alzheimer’s disease biomarkers in older adults with significant memory concern. Alzheimers Dement 11:1417–1429
Beckett LA, Donohue MC, Wang C et al (2015) The Alzheimer’s Disease Neuroimaging Initiative phase 2: increasing the length, breadth, and depth of our understanding. Alzheimers Dement 11:823–831
Saykin AJ, Wishart HA, Rabin LA et al (2006) Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology 67:834–842
Jenkinson M, Bannister P, Brady M, Smith S (2002) Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17:825–841
Toppi J, De Vico FF, Vecchiato G et al (2012) How the statistical validation of functional connectivity patterns can prevent erroneous definition of small-world properties of a brain connectivity network. Comput Math Methods 2012:130985
Wang H, Jin X, Zhang Y, Wang J (2016) Single-subject morphological brain networks: connectivity mapping, topological characterization and test-retest reliability. Brain Behav 6:e00448
Shu N, Liu Y, Li K et al (2011) Diffusion tensor tractography reveals disrupted topological efficiency in white matter structural networks in multiple sclerosis. Cereb Cortex 21:2565–2577
Li W, Zhang L, Qiao L, Shen D (2019) Towards a better estimation of functional brain network for mild cognitive impairment identification: a transfer learning View. IEEE J Biomed Health Inform 24:1160–1168
Li W, Qiao L, Zhang L, Wang Z, Shen D (2019) Functional brain network estimation with time series self-scrubbing. IEEE J Biomed Health Inform 23:2494–2504
Wen H, Liu Y, Rekik I et al (2017) Disrupted topological organization of structural networks revealed by probabilistic diffusion tractography in Tourette syndrome children. Hum Brain Mapp 38:3988–4008
Wen H, Liu Y, Rekik I et al (2017) Multi-modal multiple kernel learning for accurate identification of Tourette syndrome children. Pattern Recogn 63:601–611
Dyrba M, Grothe M, Kirste T, Teipel SJ (2015) Multimodal analysis of functional and structural disconnection in Alzheimer's disease using multiple kernel SVM. Hum Brain Mapp 36(6):2118–2131
Dosenbach NU, Nardos B, Cohen AL et al (2010) Prediction of individual brain maturity using fMRI. Science 329:1358–1361
Zhao W, Guo S, Linli Z, Yang AC, Lin CP, Tsai SJ (2020) Functional, anatomical, and morphological networks highlight the role of basal ganglia-thalamus-cortex circuits in schizophrenia. Schizophr Bull 46:422–431
Gaubert S, Raimondo F, Houot M et al (2019) EEG evidence of compensatory mechanisms in preclinical Alzheimer's disease. Brain 142:2096–2112
Sperling RA, Jack CR Jr, Aisen PS (2011) Testing the right target and right drug at the right stage. Sci Transl Med 3:111 cm33
Raichle ME (2015) The brain’s default mode network. Annu Rev Neurosci 38:433–447
Zott B, Busche MA, Sperling RA, Konnerth A (2018) What happens with the circuit in Alzheimer’s disease in mice and humans? Annu Rev Neurosci 41:277–297
Zhao Y, Raichle ME, Wen J et al (2017) In vivo detection of microstructural correlates of brain pathology in preclinical and early Alzheimer Disease with magnetic resonance imaging. Neuroimage 148:296–304
Gifford KA, Liu D, Damon SM et al (2015) Subjective memory complaint only relates to verbal episodic memory performance in mild cognitive impairment. J Alzheimers Dis 44:309–318
Jeon Y, Kim B, Kim JE et al (2016) Effects of ganglioside on working memory and the default mode network in individuals with subjective cognitive impairment: a randomized controlled trial. Am J Chin Med 44:489–514
Rodda J, Dannhauser T, Cutinha DJ, Shergill SS, Walker Z (2011) Subjective cognitive impairment: functional MRI during a divided attention task. Eur Psychiatry 26:457–462
Tong T, Gao Q, Guerrero R et al (2017) A novel grading biomarker for the prediction of conversion from mild cognitive impairment to Alzheimer’s disease. IEEE Trans Biomed Eng 64(1):155–165
Kinkingnéhun S, Sarazin M, Lehéricy S, Guichart-Gomez E, Hergueta T, Dubois B (2008) VBM anticipates the rate of progression of Alzheimer disease: a 3-year longitudinal study. Neurology 70:2201–2211
Menon V, Uddin LQ (2010) Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct 214:655–667
Menon V (2011) Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci 15:483–506
Nasrabady SE, Rizvi B, Goldman JE, Brickman AM (2018) White matter changes in Alzheimer’s disease: a focus on myelin and oligodendrocytes. Acta Neuropathol Commun 6:22
McAleese KE, Walker L, Graham S et al (2017) Parietal white matter lesions in Alzheimer’s disease are associated with cortical neurodegenerative pathology, but not with small vessel disease. Acta Neuropathol 134:459–473
Yan T, Wang Y, Weng Z et al (2019) Early-stage identification and pathological development of Alzheimer’s disease using multimodal MRI. J Alzheimers Dis 68:1013–1027
Miebach L, Wolfsgruber S, Polcher A et al (2019) Which features of subjective cognitive decline are related to amyloid pathology? Findings from the DELCODE study. Alzheimers Res Ther 11:66
Perrotin A, Mormino EC, Madison CM, Hayenga AO, Jagust WJ (2012) Subjective cognition and amyloid deposition imaging: A pittsburgh Compound B positron emission tomography study in normal elderly individuals. Arch Neurol 69:223–229
Buckley RF, Schultz AP, Hedden T et al (2017) Functional network integrity presages cognitive decline in preclinical Alzheimer disease. Neurology 89:29–37
Acknowledgements
Data collection and sharing for this project was funded by the ADNI (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, and the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd. and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Funding
This work was supported partly by grants from the National Natural Science Foundation of China (No. 81822013; 82071186), the Jiangsu Provincial Key Medical Talents (No. ZDRCA2016085), the Key Research and Development Program of Jiangsu Province of China (BE2016610), the National Key Research and Development Program of China (2016YFC1300500-504), and the Jiangsu Province Key Medical Discipline (ZDXKA2016020).
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Prof. Feng Bai.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Subject data used in the preparation for this article was obtained from publicly available study samples, i.e., ADNI. Written informed consent was obtained as part of these studies.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
Subject data (SCD patients) used in the preparation for this article was obtained from publicly available study samples, i.e., ADNI, which have not been used in previous publications.
Methodology
• Retrospective
• Cross sectional study
• Multicenter study
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
aData used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in the analysis or writing of this report. A complete listing of ADNI investigators can be found at http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf.
The abbreviations of 246 ROIs are shown in Supplemental Table 1.
Supplementary Information
ESM 1
(DOC 189 kb)
Rights and permissions
About this article
Cite this article
Chen, H., Li, W., Sheng, X. et al. Machine learning based on the multimodal connectome can predict the preclinical stage of Alzheimer’s disease: a preliminary study. Eur Radiol 32, 448–459 (2022). https://doi.org/10.1007/s00330-021-08080-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-021-08080-9