Abstract
Objectives
To evaluate the prevalence of prostate cancer (PCa) of two PI-RADS version (v) 2.1 transition zone (TZ) features (PI-RADS 1 [‘nodule in nodule’] and 2 [‘homogeneous mildly hypointense area between nodules’]).
Methods
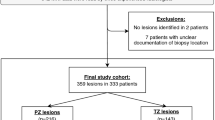
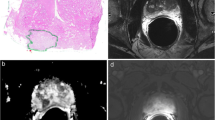
With an institutional review board approval, from a 5-year cohort between 2012 and 2017, we retrospectively identified 53 consecutive men with radical prostatectomy (RP) confirmed TZ tumors and MRI. Three blinded radiologists (R1/2/3) independently evaluated T2-weighted and diffusion-weighted imaging (DWI) using PI-RADS v2.1 for the presence of (1) ‘nodule in nodule’ (recording ‘cystic change’, inner nodule encapsulation, size, and DWI score) and (2) ‘homogeneous mildly hypointense area between nodules’ (also recording size and DWI score). MRI-RP maps established ground truth. Primary tumor was evaluated assessing PI-RADS v2.1 category, size, and presence of imaging variants.
Results
R1/2/3 identified 26/18/22 ‘nodule in nodule’ respectively with 7.7% (2/26; 95% confidence interval [95% CI]: 0.1–17.9%), 5.6% (1/18; 95% CI: 0.01–16.1%), and 4.5% (1/22; 95% CI: 0.01–13.3%) PCa (both Gleason score 3 + 4 = 7). Agreement was fair-to-substantial, kappa = 0.222–0.696. ‘Cystic change’, inner nodule absent/incomplete encapsulation and DWI score ≥ 4 for R1/R2/R2 were present in 80.8% (21/26), 46.2% (12/26), 7.7% (2/26); 94.4% (17/18), 33.3% (6/18), 5.6% (1/18); and 59.1% (13/22), 63.6% (14/22), 9.1% (2/22). Both PCa had inner nodule absent/incomplete encapsulation and DWI score ≥ 4. No other TZ tumors demonstrated ‘nodule in nodule’, nodule ‘cystic change’, or ‘homogeneous mildly hypointense area between nodules’. R1/2/3 identified 5/6/13 ‘homogeneous mildly hypointense area between nodules’ with zero PCa for any reader (upper bound 95% CI: 24.7–52.2%). Interobserver agreement was fair-to-substantial, kappa = 0.104–0.779.
Conclusion
The proportion of cancers in PI-RADS v2.1 ‘nodule in nodule’ was low (~5–8%) with zero cancers detected in ‘homogeneous mildly hypointense area between nodules’. When ‘nodule in nodule’ inner nodule shows absent or incomplete encapsulation with marked restricted diffusion, PCa may be considered; however, this warrants further studies.
Key Points
• The prevalence of clinically significant prostate cancers in PI-RADS v2.1 ‘nodule in nodule’ was low (5–8%, 95% CI: 0.1–17.9%).
• Clinically significant prostate cancer was only detected in the ‘nodule in nodule’ variant when the inner nodule showed absent or incomplete encapsulation (‘atypical nodule’) with marked restricted diffusion.
• ‘Homogeneous mildly hypointense area between nodules’ is likely benign with no cancers identified in the current study, however, with a wide 95% CI due to low prevalence.




Similar content being viewed by others
Abbreviations
- CS:
-
Clinically significant
- DWI:
-
Diffusion-weighted imaging
- ISUP:
-
International Society of Urogenital Pathology
- MP :
-
Multiparametric
- PCa:
-
Prostate cancer
- PZ:
-
Peripheral zone
- RP:
-
Radical prostatectomy
- T2W:
-
T2-weighted
- TRUS:
-
Trans-rectal ultrasound
- TZ:
-
Transition zone
References
Turkbey B, Rosenkrantz AB, Haider MA et al (2019) Prostate imaging reporting and data system version 2.1: 2019 update of Prostate Imaging Reporting and Data System Version 2. Eur Urol 76:340–351
Tran GN, Leapman MS, Nguyen HG et al (2017) Magnetic resonance imaging-ultrasound fusion biopsy during prostate cancer active surveillance. Eur Urol 72:275–281
Kasivisvanathan V, Stabile A, Neves JB et al (2019) Magnetic resonance imaging-targeted biopsy versus systematic biopsy in the detection of prostate cancer: a systematic review and meta-analysis. Eur Urol 76:284–303
Thai JN, Narayanan HA, George AK et al (2018) Validation of PI-RADS version 2 in transition zone lesions for the detection of prostate cancer. Radiology 288:485–491
Byun J, Park KJ, Kim MH, Kim JK (2020) Direct comparison of PI-RADS version 2 and 2.1 in transition zone lesions for detection of prostate cancer: preliminary experience. J Magn Reson Imaging. https://doi.org/10.1002/jmri.27080
Felker ER, Raman SS, Margolis DJ et al (2017) Risk stratification among men with prostate imaging reporting and data system version 2 category 3 transition zone lesions: is biopsy always necessary? AJR Am J Roentgenol 209:1272–1277
Greer MD, Shih JH, Lay N et al (2017) Validation of the dominant sequence paradigm and role of dynamic contrast-enhanced imaging in PI-RADS version 2. Radiology. https://doi.org/10.1148/radiol.2017161316:161316
Liddell H, Jyoti R, Haxhimolla HZ (2015) mp-MRI prostate characterised PIRADS 3 lesions are associated with a low risk of clinically significant prostate cancer - a retrospective review of 92 biopsied PIRADS 3 lesions. Curr Urol 8:96–100
Weinreb JC (2018) Organized chaos: does PI-RADS version 2 work in the transition zone? Radiology 288:492–494
Muller BG, Shih JH, Sankineni S et al (2015) Prostate cancer: interobserver agreement and accuracy with the revised prostate imaging reporting and data system at multiparametric MR imaging. Radiology 277:741–750
Wu M, Krishna S, Thornhill RE, Flood TA, McInnes MDF, Schieda N (2019) Transition zone prostate cancer: logistic regression and machine-learning models of quantitative ADC, shape and texture features are highly accurate for diagnosis. J Magn Reson Imaging 50:940–950
Chesnais AL, Niaf E, Bratan F et al (2013) Differentiation of transitional zone prostate cancer from benign hyperplasia nodules: evaluation of discriminant criteria at multiparametric MRI. Clin Radiol 68:e323–e330
Schieda N, Lim CS, Idris M et al (2017) MRI assessment of pathological stage and surgical margins in anterior prostate cancer (APC) using subjective and quantitative analysis. J Magn Reson Imaging 45:1296–1303
Stamatakis L, Siddiqui MM, Nix JW et al (2013) Accuracy of multiparametric magnetic resonance imaging in confirming eligibility for active surveillance for men with prostate cancer. Cancer 119:3359–3366
Montironi R, Hammond EH, Lin DW et al (2014) Consensus statement with recommendations on active surveillance inclusion criteria and definition of progression in men with localized prostate cancer: the critical role of the pathologist. Virchows Arch 465:623–628
Turkbey B, Mani H, Aras O et al (2012) Correlation of magnetic resonance imaging tumor volume with histopathology. J Urol 188:1157–1163
Amin MB, Lin DW, Gore JL et al (2014) The critical role of the pathologist in determining eligibility for active surveillance as a management option in patients with prostate cancer: consensus statement with recommendations supported by the College of American Pathologists, International Society of Urological Pathology, Association of Directors of Anatomic and Surgical Pathology, the New Zealand Society of Pathologists, and the Prostate Cancer Foundation. Arch Pathol Lab Med 138:1387–1405
Johnson LM, Choyke PL, Figg WD, Turkbey B (2014) The role of MRI in prostate cancer active surveillance. Biomed Res Int 2014:203906
Krishna S, Lim CS, McInnes MD et al (2017) Evaluation of MRI for diagnosis of extraprostatic extension in prostate cancer. J Magn Reson Imaging. https://doi.org/10.1002/jmri.25729
Rozenberg R, Thornhill RE, Flood TA, Hakim SW, Lim C, Schieda N (2016) Whole-tumor quantitative apparent diffusion coefficient histogram and texture analysis to predict gleason score upgrading in intermediate-risk 3 + 4 = 7 Prostate Cancer. AJR Am J Roentgenol 206:775–782
Weinreb JC, Barentsz JO, Choyke PL et al (2016) PI-RADS prostate imaging - reporting and data system: 2015, Version 2. Eur Urol 69:16–40
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174
Schieda N, van der Pol CB, Walker D et al (2020) Adverse events to the gadolinium-based contrast agent gadoxetic acid: systematic review and meta-Analysis. Radiology. https://doi.org/10.1148/radiol.2020200073:200073
Lim CS, Abreu-Gomez J, Carrion I, Schieda N (2020) Prevalence of prostate cancer in PI-RADS version 2.1 transition zone ‘atypical nodules’ upgraded by abnormal diffusion weighted imaging: correlation with MRI-directed TRUS-guided targeted biopsy. AJR Am J Roentgenol. https://doi.org/10.2214/ajr.20.23932
Costa DN, Jia L, Subramanian N et al (2020) Prospectively-reported PI-RADS version 2.1 atypical benign prostatic hyperplasia nodules with marked restricted diffusion (‘2+1’ transition zone lesions): clinically significant prostate cancer detection rates on multiparametric MRI. AJR Am J Roentgenol. https://doi.org/10.2214/ajr.20.24370
Rosenkrantz AB, Meng X, Ream JM et al (2016) Likert score 3 prostate lesions: association between whole-lesion ADC metrics and pathologic findings at MRI/ultrasound fusion targeted biopsy. J Magn Reson Imaging 43:325–332
Mussi TC, Yamauchi FI, Tridente CF et al (2020) Interobserver agreement of PI-RADS v. 2 lexicon among radiologists with different levels of experience. J Magn Reson Imaging 51:593–602
Padhani AR, Barentsz J, Villeirs G et al (2019) PI-RADS Steering Committee: the PI-RADS multiparametric MRI and MRI-directed biopsy pathway. Radiology 292:464–474
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Christopher Lim.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
The patient cohort was derived from a previous study evaluated by our group (list below); however, evaluation of PI-RADS version 2.1 ‘nodule in nodule’ and ‘homogeneous mildly hypointense area between nodules’ has not been previously studied.
Wu M, Krishna S, Thornhill RE, Flood TA, McInnes MDF, Schieda N (2019) Transition zone prostate cancer: logistic regression and machine-learning models of quantitative ADC, shape and texture features are highly accurate for diagnosis. J Magn Reson Imaging 50:940–950
Schieda N, Lim CS, Idris M et al (2017) MRI assessment of pathological stage and surgical margins in anterior prostate cancer (APC) using subjective and quantitative analysis. J Magn Reson Imaging 45:1296–1303
Methodology
• Retrospective
• Diagnostic study
• Performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lim, C.S., Abreu-Gomez, J., Flood, T.A. et al. Prevalence of prostate cancer in PI-RADS version 2.1 T2-weighted transition zone ‘nodule in nodule’ and ‘homogeneous mildly hypointense area between nodules’ criteria: MRI-radical prostatectomy histopathological evaluation. Eur Radiol 31, 7792–7801 (2021). https://doi.org/10.1007/s00330-021-07855-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-021-07855-4