Abstract
Objectives
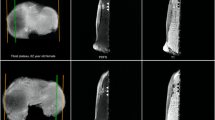
To evaluate the reliability and validity of measuring subchondral trabecular biomarkers in “conventional” intermediate-weighted (IW) MRI sequences and to assess the predictive value of biomarker changes for predicting near-term symptomatic and structural progressions in knee osteoarthritis (OA).
Methods
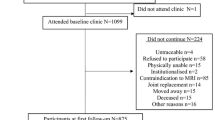
For this study, a framework for measuring trabecular biomarkers in the proximal medial tibia in the “conventional” IW MRI sequence was developed. The reliability of measuring these biomarkers (trabecular thickness [cTbTh], spacing [cTbSp], connectivity density [cConnD], and bone-to-total volume ratio [cBV/TV]) was evaluated in the Bone Ancillary Study (within the Osteoarthritis Initiative [OAI]). The validity of these measurements was assessed by comparing to “apparent” biomarkers (from high-resolution steady-state MRI sequence) and peri-articular bone marrow density (BMD, from dual-energy X-ray absorptiometry). The association of these biomarker changes from baseline to 24 months (using the Reliable Change Index) with knee OA progression was studied in the FNIH OA Biomarkers Consortium (within the OAI). Pain and radiographic progression were evaluated by comparing baseline WOMAC pain score and radiographic joint space width with the 24-to-48-month scores/measurements. Associations between biomarker changes and these outcomes were studied using logistic regression adjusted for the relevant covariates.
Results
With acceptable reliability, the cTbTh and cBV/TV, but not cTbSp or cConnD, were modestly associated with the “apparent” biomarkers and peri-articular BMD (β: 1.10 [95% CI: 0.45–1.75], p value: 0.001 and β: 3.69 [95% CI: 2.56–4.83], p value: < 0.001, respectively). Knees with increased cTbTh had higher (OR: 1.44 [95% CI: 1.03–2.02], p value: 0.035) and knees with decreased cTbTh (OR: 0.69 [95% CI: 0.49–0.95], p value: 0.026) or decreased cBV/TV (OR: 0.67 [95% CI: 0.48–0.93], p value: 0.018) had lower odds of experiencing OA pain progression over the follow-ups.
Conclusions
Measurement of certain “conventional” MRI-based subchondral trabecular biomarkers has high reliability and modest validity. Though modest, there are significant associations between these biomarker changes and knee OA pain progression up to 48-month follow-up.
Key Points
• Despite the lower spatial resolution than what is required to accurately study the subchondral trabecular microstructures, the “conventional” IW MRI sequences may retain adequate information that allows quantification of trabecular microstructure biomarkers.
• Subchondral trabecular biomarkers obtained from “conventional” IW MRI sequences (i.e., cTbTh, cTbSp, and cBV/TV) are reliable and valid measures of trabecular microstructure changes compared to those from “apparent” trabecular biomarkers (from the FISP MRI sequence) and peri-articular BMD (from DXA).
• Increased trabecular thickness and bone-to-total ratio (cTbTh and cBV/TV, obtained from “conventional” IW MRI sequences) from baseline to 24-month visits may be associated with higher odds of knee OA pain progression over 48 months of follow-up.



Similar content being viewed by others
Data availability
The de-identified clinical and demographic information of subjects is publically available at the OAI project data repository at https://oai.nih.gov. The codes for the image segmentation and analysis framework, the dataset of subchondral trabecular biomarker measurements, and the R codes used in this work are available from the corresponding author upon reasonable requests.
Abbreviations
- μCT:
-
Micro-computed tomography
- BMI:
-
Body mass index
- BV/TV:
-
Bone-to-total volume ratio
- CI:
-
Confidence interval
- ConnD:
-
Connectivity density
- CT:
-
Computed tomography
- DXA:
-
Dual-energy X-ray absorptiometry
- FISP:
-
3D fast imaging with steady-state precession
- FNIH:
-
Foundation for the National Institutes of Health
- HR-QCT:
-
High-resolution quantitative computed tomography
- ICC:
-
Intraclass correlation coefficient
- IW:
-
Intermediate-weighted
- JSW:
-
Joint space width
- KLG:
-
Kellgren and Lawrence grade
- MRI:
-
Magnetic resonance imaging
- OA:
-
Osteoarthritis
- OAI:
-
Osteoarthritis Initiative
- OR:
-
Odds ratio
- RCI:
-
Reliable Change Index
- ROIs:
-
Regions of interest
- TbSp:
-
Trabecular spacing
- TbTh:
-
Trabecular thickness
- WOMAC:
-
Western Ontario and McMaster Universities Osteoarthritis Index
References
GBD 2017 Disease and Injury Incidence and Prevalence Collaborator (2018) Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet 392:1789–1858
Runhaar J, Zhang Y (2018) Can we prevent OA? Epidemiology and public health insights and implications. Rheumatology (Oxford) 57:iv3–iv9
Kraus VB, Collins JE, Charles HC et al (2018) Predictive validity of radiographic trabecular bone texture in knee osteoarthritis: the Osteoarthritis Research Society International/Foundation for the National Institutes of Health Osteoarthritis Biomarkers Consortium. Arthritis Rheumatol 70:80–87
Zhu S, Zhu J, Zhen G et al (2019) Subchondral bone osteoclasts induce sensory innervation and osteoarthritis pain. J Clin Invest 129:1076–1093
Lo GH, Schneider E, Driban JB et al (2018) Periarticular bone predicts knee osteoarthritis progression: data from the Osteoarthritis Initiative. Semin Arthritis Rheum 48:155–161
MacKay JW, Kapoor G, Driban JB et al (2018) Association of subchondral bone texture on magnetic resonance imaging with radiographic knee osteoarthritis progression: data from the Osteoarthritis Initiative Bone Ancillary Study. Eur Radiol 28:4687–4695
Lowitz T, Museyko O, Bousson V et al (2017) Advanced Knee Structure Analysis (AKSA): a comparison of bone mineral density and trabecular texture measurements using computed tomography and high-resolution peripheral quantitative computed tomography of human knee cadavers. Arthritis Res Ther 19:1
Adams JE (2013) Advances in bone imaging for osteoporosis. Nat Rev Endocrinol 9:28–42
Burghardt AJ, Link TM, Majumdar S (2011) High-resolution computed tomography for clinical imaging of bone microarchitecture. Clin Orthop Relat Res 469:2179–2193
Graeff C, Campbell GM, Peña J et al (2015) Administration of romosozumab improves vertebral trabecular and cortical bone as assessed with quantitative computed tomography and finite element analysis. Bone 81:364–369
Bolbos RI, Zuo J, Banerjee S et al (2008) Relationship between trabecular bone structure and articular cartilage morphology and relaxation times in early OA of the knee joint using parallel MRI at 3 T. Osteoarthritis Cartilage 16:1150–1159
Hafezi-Nejad N, Guermazi A, Roemer FW et al (2017) Prediction of medial tibiofemoral compartment joint space loss progression using volumetric cartilage measurements: data from the FNIH OA Biomarkers Consortium. Eur Radiol 27:464–473
Kraus VB, Collins JE, Hargrove D et al (2017) Predictive validity of biochemical biomarkers in knee osteoarthritis: data from the FNIH OA Biomarkers Consortium. Ann Rheum Dis 76:186–195
Kraus VB, Hargrove DE, Hunter DJ, Renner JB, Jordan JM (2017) Establishment of reference intervals for osteoarthritis-related soluble biomarkers: the FNIH/OARSI OA Biomarkers Consortium. Ann Rheum Dis 76:179–185
Hunter D, Nevitt M, Lynch J et al (2016) Longitudinal validation of periarticular bone area and 3D shape as biomarkers for knee OA progression? Data from the FNIH OA Biomarkers Consortium. Ann Rheum Dis 75:1607–1614
Sezgin M, Sankur B (2004) Survey over image thresholding techniques and quantitative performance evaluation. J Electron Imaging 13:146–165
Doube M, Kłosowski MM, Arganda-Carreras I et al (2010) BoneJ: free and extensible bone image analysis in ImageJ. Bone 47:1076–1079
Haj-Mirzaian A, Guermazi A, Hafezi-Nejad N et al (2018) Superolateral Hoffa’s fat pad (SHFP) oedema and patellar cartilage volume loss: quantitative analysis using longitudinal data from the Foundation for the National Institute of Health (FNIH) Osteoarthritis Biomarkers Consortium. Eur Radiol 28:4134–4145
Wise EA (2004) Methods for analyzing psychotherapy outcomes: a review of clinical significance, reliable change, and recommendations for future directions. J Pers Assess 82:50–59
Chiba K, Uetani M, Kido Y et al (2012) Osteoporotic changes of subchondral trabecular bone in osteoarthritis of the knee: a 3-T MRI study. Osteoporos Int 23:589–597
Bedson J, Croft PR (2008) The discordance between clinical and radiographic knee osteoarthritis: a systematic search and summary of the literature. BMC Musculoskelet Disord 9:116
Finan PH, Buenaver LF, Bounds SC et al (2013) Discordance between pain and radiographic severity in knee osteoarthritis: findings from quantitative sensory testing of central sensitization. Arthritis Rheum 65:363–372
Neogi T, Felson D, Niu J et al (2009) Association between radiographic features of knee osteoarthritis and pain: results from two cohort studies. BMJ 339:b2844
Birch CE, Mensch KS, Desarno MJ, Beynnon BD, Tourville TW (2018) Subchondral trabecular bone integrity changes following ACL injury and reconstruction: a cohort study with a nested, matched case-control analysis. Osteoarthritis Cartilage 26:762–769
Lo GH, Niu J, McLennan CE et al (2008) Meniscal damage associated with increased local subchondral bone mineral density: a Framingham study. Osteoarthritis Cartilage 16:261–267
Sharma AR, Jagga S, Lee SS, Nam JS (2013) Interplay between cartilage and subchondral bone contributing to pathogenesis of osteoarthritis. Int J Mol Sci 14:19805–19830
Burr DB (2005) Increased biological activity of subchondral mineralized tissues underlies the progressive deterioration of articular cartilage in osteoarthritis. J Rheumatol 32:1156–1158 discussion 1158-1159
Aso K, Shahtaheri SM, Hill R, Wilson D, McWilliams DF, Walsh DA (2019) Associations of symptomatic knee osteoarthritis with histopathologic features in subchondral bone. Arthritis Rheumatol 71:916–924
Marques J, Genant HK, Lillholm M, Dam EB (2013) Diagnosis of osteoarthritis and prognosis of tibial cartilage loss by quantification of tibia trabecular bone from MRI. Magn Reson Med 70:568–575
Newitt DC, van Rietbergen B, Majumdar S (2002) Processing and analysis of in vivo high-resolution MR images of trabecular bone for longitudinal studies: reproducibility of structural measures and micro-finite element analysis derived mechanical properties. Osteoporos Int 13:278–287
Hildebrand T, Rüegsegger P (1997) A new method for the model-independent assessment of thickness in three-dimensional images. J Microsc 185:67–75
Thomsen JS, Laib A, Koller B, Prohaska S, Mosekilde L, Gowin W (2005) Stereological measures of trabecular bone structure: comparison of 3D micro computed tomography with 2D histological sections in human proximal tibial bone biopsies. J Microsc 218:171–179
Acknowledgments
The OAI was a collaborative project between the public and private sectors. This project included the five contracts N01-AR-2-2258, N01-AR-2-2259, N01-AR-2-2260, N01-AR-2-2261, and N01-AR-2-2262 and was conducted by the OAI project investigators. The OAI was financially supported by the National Institutes of Health (NIH). Private funding partners were Merck Research Laboratories, Novartis Pharmaceuticals Corporation, GlaxoSmithKline, and Pfizer, Inc.
In preparing this manuscript, OAI project publicly available datasets were used, and the results of this work do not necessarily reflect the opinions of the OAI project investigators, the NIH, or the private funding partners.
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Shadpour Demehri MD (Johns Hopkins University).
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
One of the authors has significant statistical expertise.
Informed consent
Subjects have given informed consent before participating in the OAI project.
Ethical approval
The medical ethics review boards of the University of California, San Francisco (Approval Number: 10-00532) and the four OAI project clinical centers recognized the project as Health Insurance Portability and Accountability Act (HIPAA)–compliant.
Methodology
• Retrospective
• Case-control study
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supporting information
ESM 1
(DOCX 604 kb)
Rights and permissions
About this article
Cite this article
Pishgar, F., Guermazi, A., Roemer, F.W. et al. Conventional MRI-based subchondral trabecular biomarkers as predictors of knee osteoarthritis progression: data from the Osteoarthritis Initiative. Eur Radiol 31, 3564–3573 (2021). https://doi.org/10.1007/s00330-020-07512-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-07512-2