Abstract
Objectives
Preoperative differentiation between benign parotid gland tumors (BPGT) and malignant parotid gland tumors (MPGT) is important for treatment decisions. The purpose of this study was to develop and validate an MRI-based radiomics nomogram for the preoperative differentiation of BPGT from MPGT.
Methods
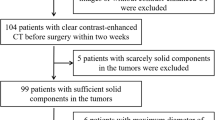
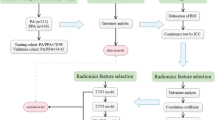
A total of 115 patients (80 in training set and 35 in external validation set) with BPGT (n = 60) or MPGT (n = 55) were enrolled. Radiomics features were extracted from T1-weighted and fat-saturated T2-weighted images. A radiomics signature model and a radiomics score (Rad-score) were constructed and calculated. A clinical-factors model was built based on demographics and MRI findings. A radiomics nomogram model combining the Rad-score and independent clinical factors was constructed using multivariate logistic regression analysis. The diagnostic performance of the three models was evaluated and validated using ROC curves on the training and validation datasets.
Results
Seventeen features from MR images were used to build the radiomics signature. The radiomics nomogram incorporating the clinical factors and radiomics signature had an AUC value of 0.952 in the training set and 0.938 in the validation set. Decision curve analysis showed that the nomogram outperformed the clinical-factors model in terms of clinical usefulness.
Conclusions
The above-described radiomics nomogram performed well for differentiating BPGT from MPGT, and may help in the clinical decision-making process.
Key Points
• Differential diagnosis between BPGT and MPGT is rather difficult by conventional imaging modalities.
• A radiomics nomogram integrated with the radiomics signature, clinical data, and MRI features facilitates differentiation of BPGT from MPGT with improved diagnostic efficacy.






Similar content being viewed by others
Abbreviations
- 3-D:
-
Three-dimensional
- ANOVA:
-
Analysis of variance
- BPGT:
-
Benign parotid gland tumors
- CI:
-
Confidence interval
- DCA:
-
Decision curve analysis
- DLI:
-
Deep lobe involved
- FNA:
-
Fine needle aspiration
- fs-T2WI:
-
Fat-saturated T2-weighted images
- GLCM:
-
Gray-level co-occurrence matrix
- GLDM:
-
Gray-level dependence matrix
- GLRLM:
-
Gray-level run length matrix
- GLSZM:
-
Gray-level size zone matrix
- ICC:
-
Inter-/intra-class correlation coefficient
- IST:
-
Infiltration of surrounding tissue
- LASSO:
-
Least absolute shrinkage and selection operator
- MPGT:
-
Malignant parotid gland tumors
- NGTDM:
-
Neighboring gray-tone difference matrix
- Nomo-score:
-
Nomogram score
- OR:
-
Odds ratio
- Rad-score:
-
Radiomics score
- SI:
-
Signal intensity
- T1WI:
-
T1-Weighted images
References
Stenner M, Klussmann JP (2009) Current update on established and novel biomarkers in salivary gland carcinoma pathology and the molecular pathways involved. Eur Arch Otorhinolaryngol 266:333–341
Bussu F, Parrilla C, Rizzo D, Almadori G, Paludetti G, Galli J (2011) Clinical approach and treatment of benign and malignant parotid masses, personal experience. Acta Otorhinolaryngol Ital 31:135–143
Papadogeorgakis N, Skouteris CA, Mylonas AI, Angelopoulos AP (2004) Superficial parotidectomy: technical modifications based on tumour characteristics. J Craniomaxillofac Surg 32:350–353
Zbaren P, Schar C, Hotz MA, Loosli H (2001) Value of fine-needle aspiration cytology of parotid gland masses. Laryngoscope 111:1989–1992
Sergi B, Contucci AM, Corina L, Paludetti G (2004) Value of fine-needle aspiration cytology of parotid gland masses. Laryngoscope 114:789
Bhatia KS, Rasalkar DD, Lee YP et al (2010) Evaluation of real-time qualitative sonoelastography of focal lesions in the parotid and submandibular glands: applications and limitations. Eur Radiol 20:1958–1964
Lee YY, Wong KT, King AD, Ahuja AT (2008) Imaging of salivary gland tumours. Eur J Radiol 66:419–436
Habermann CR, Arndt C, Graessner J et al (2009) Diffusion-weighted echo-planar MR imaging of primary parotid gland tumors: is a prediction of different histologic subtypes possible? AJNR Am J Neuroradiol 30:591–596
Gillies RJ, Kinahan PE, Hricak H (2016) Radiomics: images are more than pictures, they are data. Radiology 278:563–577
Lambin P, Rios-Velazquez E, Leijenaar R et al (2012) Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer 48:441–446
Buch K, Fujita A, Li B, Kawashima Y, Qureshi MM, Sakai O (2015) Using texture analysis to determine human papillomavirus status of oropharyngeal squamous cell carcinomas on CT. AJNR Am J Neuroradiol 36:1343–1348
Al Ajmi E, Forghani B, Reinhold C, Bayat M, Forghani R (2018) Spectral multi-energy CT texture analysis with machine learning for tissue classification: an investigation using classification of benign parotid tumours as a testing paradigm. Eur Radiol 28:2604–2611
Fruehwald-Pallamar J, Czerny C, Holzer-Fruehwald L et al (2013) Texture-based and diffusion-weighted discrimination of parotid gland lesions on MR images at 3.0 Tesla. NMR Biomed 26:1372–1379
Christe A, Waldherr C, Hallett R, Zbaeren P, Thoeny H (2011) MR imaging of parotid tumors: typical lesion characteristics in MR imaging improve discrimination between benign and malignant disease. AJNR Am J Neuroradiol 32:1202–1207
Collewet G, Strzelecki M, Mariette F (2004) Influence of MRI acquisition protocols and image intensity normalization methods on texture classification. Magn Reson Imaging 22:81–91
Tustison NJ, Avants BB, Cook PA et al (2010) N4ITK: improved N3 bias correction. IEEE Trans Med Imaging 29:1310–1320
Depeursinge A, Foncubierta-Rodriguez A, Van De Ville D, Muller H (2014) Three-dimensional solid texture analysis in biomedical imaging: review and opportunities. Med Image Anal 18:176–196
Alhamzawi R, Ali HTM (2018) The Bayesian adaptive lasso regression. Math Biosci 303:75–82
Okahara M, Kiyosue H, Hori Y, Matsumoto A, Mori H, Yokoyama S (2003) Parotid tumors: MR imaging with pathological correlation. Eur Radiol 13(Suppl 4):L25–L33
Takashima S, Wang J, Takayama F et al (2001) Parotid masses: prediction of malignancy using magnetization transfer and MR imaging findings. AJR Am J Roentgenol 176:1577–1584
Zhang YF, Li H, Wang XM, Cai YF (2019) Sonoelastography for differential diagnosis between malignant and benign parotid lesions: a meta-analysis. Eur Radiol 29:725–735
Hwang JH, Kim DW, Kim KS, Lee SY (2019) Mucosa-associated lymphoid tissue lymphoma of the accessory parotid gland presenting as a simple cheek mass: a case report. Medicine (Baltimore) 98:e17042
Schmidt RL, Hall BJ, Wilson AR, Layfield LJ (2011) A systematic review and meta-analysis of the diagnostic accuracy of fine-needle aspiration cytology for parotid gland lesions. Am J Clin Pathol 136:45–59
Cantisani V, David E, De Virgilio A et al (2017) Prospective evaluation of quasistatic ultrasound elastography (USE) compared with baseline US for parotid gland lesions: preliminary results of elasticity contrast index (ECI) evaluation. Med Ultrason 19:32–38
Mansour N, Bas M, Stock KF, Strassen U, Hofauer B, Knopf A (2017) Multimodal ultrasonographic pathway of parotid gland lesions. Ultraschall Med 38:166–173
Altinbas NK, Gundogdu Anamurluoglu E, Oz II et al (2017) Real-time sonoelastography of parotid gland tumors. J Ultrasound Med 36:77–87
Yabuuchi H, Fukuya T, Tajima T, Hachitanda Y, Tomita K, Koga M (2003) Salivary gland tumors: diagnostic value of gadolinium-enhanced dynamic MR imaging with histopathologic correlation. Radiology 226:345–354
Niazi M, Mohammadzadeh M, Aghazadeh K et al (2020) Perfusion computed tomography scan imaging in differentiation of benign from malignant parotid lesions. Int Arch Otorhinolaryngol 24:e160–e169
Jin GQ, Su DK, Xie D, Zhao W, Liu LD, Zhu XN (2011) Distinguishing benign from malignant parotid gland tumours: low-dose multi-phasic CT protocol with 5-minute delay. Eur Radiol 21:1692–1698
Morcos SK, Thomsen HS, Webb JA (1999) Contrast-media-induced nephrotoxicity: a consensus report. Contrast Media Safety Committee, European Society of Urogenital Radiology (ESUR). Eur Radiol 9:1602–1613
Thomsen HS, Morcos SK, Almen T et al (2013) Nephrogenic systemic fibrosis and gadolinium-based contrast media: updated ESUR Contrast Medium Safety Committee guidelines. Eur Radiol 23:307–318
Takumi K, Fukukura Y, Hakamada H, Ideue J, Kumagae Y, Yoshiura T (2017) Value of diffusion tensor imaging in differentiating malignant from benign parotid gland tumors. Eur J Radiol 95:249–256
Zhang Z, Song C, Zhang Y, Wen B, Zhu J, Cheng J (2019) Apparent diffusion coefficient (ADC) histogram analysis: differentiation of benign from malignant parotid gland tumors using readout-segmented diffusion-weighted imaging. Dentomaxillofac Radiol 48:20190100
Yabuuchi H, Matsuo Y, Kamitani T et al (2008) Parotid gland tumors: can addition of diffusion-weighted MR imaging to dynamic contrast-enhanced MR imaging improve diagnostic accuracy in characterization? Radiology 249:909–916
Milad P, Elbegiermy M, Shokry T et al (2017) The added value of pretreatment DW MRI in characterization of salivary glands pathologies. Am J Otolaryngol 38:13–20
Nie P, Yang G, Wang Z et al (2020) A CT-based radiomics nomogram for differentiation of renal angiomyolipoma without visible fat from homogeneous clear cell renal cell carcinoma. Eur Radiol 30:1274–1284
Wang H, Nie P, Wang Y et al (2020) Radiomics nomogram for differentiating between benign and malignant soft-tissue masses of the extremities. J Magn Reson Imaging 51:155–163
Wang H, Chen H, Duan S, Hao D, Liu J (2020) Radiomics and machine learning with multiparametric preoperative MRI may accurately predict the histopathological grades of soft tissue sarcomas. J Magn Reson Imaging 51:791–797
Fruehwald-Pallamar J, Hesselink JR, Mafee MF, Holzer-Fruehwald L, Czerny C, Mayerhoefer ME (2016) Texture-based analysis of 100 MR examinations of head and neck tumors - is it possible to discriminate between benign and malignant masses in a multicenter trial? Rofo 188:195–202
Acknowledgments
We thank Nicole Okoh, PhD, from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Wen-jian Xu.
Conflict of interest
The authors declare that they have no conflict of interest.
Statistics and biometry
One of the authors (Jian Li) has significant statistical expertise.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• diagnostic study/observational
• multicenter study
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ying-mei Zheng and Jian Li are collaborative first author
Rights and permissions
About this article
Cite this article
Zheng, Ym., Li, J., Liu, S. et al. MRI-Based radiomics nomogram for differentiation of benign and malignant lesions of the parotid gland. Eur Radiol 31, 4042–4052 (2021). https://doi.org/10.1007/s00330-020-07483-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-07483-4