Abstract
Objectives
Temporal muscle thickness (TMT) is a surrogate marker of sarcopenia, correlated with survival expectancy in patients suffering from brain metastases and recurrent or treated glioblastoma. We evaluated the prognostic relevance of TMT measured on brain MRIs acquired at diagnosis in patients affected by glioblastoma.
Methods
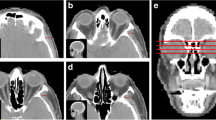
We retrospectively enrolled 51 patients in our Institution affected by methylated MGMT promoter, IDH1–2 wild-type glioblastoma, who underwent complete surgical resection and subsequent radiotherapy with concomitant and maintenance temozolomide, from January 1, 2015, to April 30, 2017. The last clinical/radiological follow-up date was set to September 3, 2019. TMT was measured bilaterally on reformatted post-contrast 3D MPRAGE images, acquired on our 3-T scanner no more than 2 days before surgery. The median, 25th, and 75th percentile TMT values were identified and population was subdivided accordingly; afterwards, statistical analyses were performed to verify the association among overall survival (OS) and TMT, sex, age, and ECOG performance status.
Results
In our cohort, the median OS was 20 months (range 3–51). Patients with a TMT ≥ 8.4 mm (median value) did not show a statistically significant increase in OS (Cox regression model: HR 1.34, 95% CI 0.68–2.63, p = 0.403). Similarly, patients with a TMT ≥ 9.85 mm (fourth quartile) did not differ in OS compared to those with TMT ≤ 7 mm (first quartile). The statistical analyses confirmed a significant association among TMT and sex (p = 0.0186), but none for age (p = 0.642) and performance status (p = 0.3982).
Conclusions
In our homogeneous cohort of patients with glioblastoma at diagnosis, TMT was not associated with prognosis, age, or ECOG performance status.
Key Points
• Temporal muscle thickness (TMT) is a surrogate marker of sarcopenia and has been correlated with survival expectancy in patients suffering from brain metastases and recurrent or treated glioblastoma.
• We appraised the correlation among TMT and survival, sex, age at surgery, and performance status, measured on brain MRIs of patients affected by glioblastoma at diagnosis.
• TMT did not show any significant correlation with prognosis, age at surgery, or performance status, and its usefulness might be restricted only to patients with brain metastases and recurrent or treated glioblastoma.



Similar content being viewed by others
Abbreviations
- ECOG:
-
Eastern Cooperative Oncology Group
- GBM:
-
Glioblastoma
- IDH:
-
Isocitrate dehydrogenase
- MGMT:
-
O6-methylguanine-DNA methyltransferase
- MPRAGE:
-
Magnetization-prepared rapid acquisition with gradient echo
- OS:
-
Overall survival
- PS:
-
Performance status
- RT:
-
Radiotherapy
- SMI:
-
Skeletal mass index
- TMT:
-
Temporal muscle thickness
- TMZ:
-
Temozolomide
References
Hanif F, Muzaffar K, Perveen K, Malhi SM, Simjee SHU (2017) Glioblastoma multiforme: a review of its epidemiology and pathogenesis through clinical presentation and treatment. Asian Pac J Cancer Prev 18:3–9. https://doi.org/10.22034/APJCP.2017.18.1.3
Tykocki T, Eltayeb M (2018) Ten-year survival in glioblastoma. A systematic review. J Clin Neurosci 54:7–13. https://doi.org/10.1016/j.jocn.2018.05.002
Chaichana KL, Martinez-Gutierrez JC, De la Garza-Ramos R et al (2013) Factors associated with survival for patients with glioblastoma with poor pre-operative functional status. J Clin Neurosci 20:818–823. https://doi.org/10.1016/j.jocn.2012.07.016
Cruz-Jentoft AJ, Bahat G, Bauer J et al (2019) Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 48:16–31. https://doi.org/10.1093/ageing/afy169
Lee HS, Kim SY, Chung MJ et al (2019) Skeletal muscle mass predicts poor prognosis in patients with advanced pancreatic cancer undergoing second-line FOLFIRINOX chemotherapy. Nutr Cancer 71:1100–1107. https://doi.org/10.1080/01635581.2019.1597906
Limpawattana P, Theerakulpisut D, Wirasorn K, Sookprasert A, Khuntikeo N, Chindaprasirt J (2018) The impact of skeletal muscle mass on survival outcome in biliary tract cancer patients. PLoS One 13:e0204985. https://doi.org/10.1371/journal.pone.0204985
Penna F, Ballarò R, Beltrà M, De Lucia S, Castillo LG, Costelli P (2019) The skeletal muscle as an active player against cancer cachexia. Front Physiol 10:41. https://doi.org/10.3389/fphys.2019.00041
Leitner J, Pelster S, Schöpf V et al (2018) High correlation of temporal muscle thickness with lumbar skeletal muscle cross-sectional area in patients with brain metastases. PLoS One 13:e0207849. https://doi.org/10.1371/journal.pone.0207849
Hasegawa Y, Yoshida M, Sato A et al (2019) Temporal muscle thickness as a new indicator of nutritional status in older individuals. Geriatr Gerontol Int 19:135–140. https://doi.org/10.1111/ggi.13570
Furtner J, Berghoff AS, Schöpf V et al (2018) Temporal muscle thickness is an independent prognostic marker in melanoma patients with newly diagnosed brain metastases. J Neurooncol 140:173–178. https://doi.org/10.1007/s11060-018-2948-8
Furtner J, Genbrugge E, Gorlia T et al (2019) Temporal muscle thickness is an independent prognostic marker in patients with progressive glioblastoma: translational imaging analysis of the EORTC 26101 trial. Neuro Oncol. https://doi.org/10.1093/neuonc/noz131
Hsieh K, Hwang ME, Estevez-Inoa G et al (2019) Temporalis muscle width as a measure of sarcopenia correlates with overall survival in patients with newly diagnosed glioblastoma. J Radiat Oncol 8:379–387. https://doi.org/10.1007/s13566-019-00408-9
Furtner J, Berghoff AS, Albtoush OM et al (2017) Survival prediction using temporal muscle thickness measurements on cranial magnetic resonance images in patients with newly diagnosed brain metastases. Eur Radiol 27:3167–3173. https://doi.org/10.1007/s00330-016-4707-6
Chukwueke UN, Wen PY (2019) Use of the Response Assessment in Neuro-Oncology (RANO) criteria in clinical trials and clinical practice. CNS Oncol 8:CNS28. https://doi.org/10.2217/cns-2018-0007
Kelly C, Majewska P, Ioannidis S, Raza MH, Williams M (2017) Estimating progression-free survival in patients with glioblastoma using routinely collected data. J Neurooncol 135:621–627. https://doi.org/10.1007/s11060-017-2619-1
Sayer AA, Syddall H, Martin H, Baylis D, Cooper C (2008) The developmental origins of sarcopenia. J Nutr Health Aging 12:427–432. https://doi.org/10.1007/bf02982703
Jain KK (2018) A critical overview of targeted therapies for glioblastoma. Front Oncol 8:419. https://doi.org/10.3389/fonc.2018.00419
Bauer J, Morley JE, Schols AMWJ et al (2019) Sarcopenia: a time for action. an SCWD position paper. J Cachexia Sarcopenia Muscle 10:956–961. https://doi.org/10.1002/jcsm.12483
Liguori I, Russo G, Aran L et al (2018) Sarcopenia: assessment of disease burden and strategies to improve outcomes. Clin Interv Aging 13:913–927. https://doi.org/10.2147/CIA.S149232
Faron A, Pieper CC, Schmeel FC, et al (2019) Fat-free muscle area measured by magnetic resonance imaging predicts overall survival of patients undergoing radioembolization of colorectal cancer liver metastases. Eur Radiol 1–9. https://doi.org/10.1007/s00330-018-5976-z
Cruz-Jentoft AJ, Baeyens JP, Bauer JM et al (2010) Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing 39:412–423. https://doi.org/10.1093/ageing/afq034
Chang AE, Matory YL, Dwyer AJ et al (1987) Magnetic resonance imaging versus computed tomography in the evaluation of soft tissue tumors of the extremities. Ann Surg 205:340–348. https://doi.org/10.1097/00000658-198704000-00002
Albano D, Messina C, Vitale J, Sconfienza LM (2020) Imaging of sarcopenia: old evidence and new insights. Eur Radiol 30:2199–2208. https://doi.org/10.1007/s00330-019-06573-2
Engelke K, Museyko O, Wang L, Laredo J-D (2018) Quantitative analysis of skeletal muscle by computed tomography imaging-State of the art. J Orthop Translat 15:91–103. https://doi.org/10.1016/j.jot.2018.10.004
Montano-Loza AJ, Ebadi M (2020) Definition and diagnosis of sarcopenia in the research and clinical settings. In: Tandon P, Montano-Loza AJ (eds) Frailty and sarcopenia in cirrhosis: the basics, the challenges, and the future. Springer International Publishing, Cham, pp 3–12
Grünheid T, Langenbach GEJ, Korfage JAM, Zentner A, van Eijden TMGJ (2009) The adaptive response of jaw muscles to varying functional demands. Eur J Orthod 31:596–612. https://doi.org/10.1093/ejo/cjp093
Ogawa S, Yakabe M, Akishita M (2016) Age-related sarcopenia and its pathophysiological bases. Inflamm Regen 36:17. https://doi.org/10.1186/s41232-016-0022-5
Roubenoff R (2000) Sarcopenia and its implications for the elderly. Eur J Clin Nutr 54(Suppl 3):S40–S47. https://doi.org/10.1038/sj.ejcn.1601024
Roila F, Lupattelli M, Sassi M et al (1991) Intra and interobserver variability in cancer patients’ performance status assessed according to Karnofsky and ECOG scales. Ann Oncol 2:437–439. https://doi.org/10.1093/oxfordjournals.annonc.a057981
Datta SS, Ghosal N, Daruvala R et al (2019) How do clinicians rate patient’s performance status using the ECOG performance scale? A mixed-methods exploration of variability in decision-making in oncology. Ecancermedicalscience 13:913. https://doi.org/10.3332/ecancer.2019.913
Funding
The authors state that this work has not received any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Prof. Letterio S. Politi.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
Dr. Emanuela Morenghi kindly provided statistical advice for this manuscript.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
Institutional Review Board approval was not required because of the retrospective nature of the study.
Methodology
• retrospective
• observational
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Fig. 1
X: TMT mean value of observer #1 measurements. Y: TMT mean value of observer #2 measurements. The units have to be intended in millimeters. (DOCX 92 kb)
Rights and permissions
About this article
Cite this article
Muglia, R., Simonelli, M., Pessina, F. et al. Prognostic relevance of temporal muscle thickness as a marker of sarcopenia in patients with glioblastoma at diagnosis. Eur Radiol 31, 4079–4086 (2021). https://doi.org/10.1007/s00330-020-07471-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-07471-8