Abstract
Objectives
To assess whether spatial labeling with multiple inversion pulses (SLEEK) sequence can be employed as a one-stop assessment method for evaluating renal function and displaying renal artery in hypertensive patients with suspected renal dysfunction.
Methods
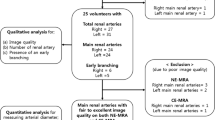
A total of 78 patients with suspected hypertensive renal damage were enrolled in this retrospective study. All patients underwent MRI examinations, and both SLEEK and DWI sequences were performed simultaneously. According to estimated glomerular filtration rate (eGFR), patients were divided into three groups (Group 1, eGFR> 90; Group 2, eGFR = 60–90; Group 3, eGFR< 60). Twenty-two of these patients also underwent CT angiography (CTA) examination. Comparison between CTA, DWI, and eGFR was performed to assess the value of SLEEK in evaluating renal function and displaying renal artery.
Results
The performance of SLEEK to display renal artery was highly consistent with the results of CTA (kappa = 0.713). The corticomedullary contrast ratio positively correlated with eGFR (p = 0.004, r = 0.322) and was significantly higher in SLEEK images than in DWI images in all three groups (p < 0.001). There was no significant difference in corticomedullary contrast ratio in SLEEK images between Group 1 and Group 2 (p = 0.285). However, the minimal renal cortical thickness, which significantly correlated with eGFR (p < 0.001, r = 0.866), was significantly different between Group 1 and Group 2 (p < 0.001). ROC analysis showed good diagnostic performance when differentiating patients with eGFR> 60 from those with eGFR< 60.
Conclusions
The SLEEK sequence could evaluate simultaneously renal function through corticomedullary differentiation and renal arteries, enabling one-stop assessment in hypertensive patients with suspected renal dysfunction.
Key Points
• Spatial labeling with multiple inversion pulses (SLEEK) improves renal corticomedullary differentiation in hypertensive patients with renal dysfunction compared with DWI.
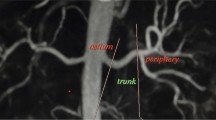
• SLEEK clearly displays renal artery in hypertensive patients with renal dysfunction.
• SLEEK could be utilized as a one-stop assessment method for evaluating renal function and renal artery in hypertensive patients.






Similar content being viewed by others
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- AUC:
-
The areas under the receiver operating characteristic curves
- BSP TI:
-
Blood-suppression inversion times
- CE-MRI:
-
Contrast-enhanced magnetic resonance imaging
- CKD:
-
Chronic kidney disease
- CTA:
-
Computed tomography angiography
- DWI:
-
Diffusion-weighted imaging
- eGFR:
-
Estimated glomerular filtration rate
- ICC:
-
Intraclass correlation coefficient
- ROC:
-
Receiver operating characteristic
- ROI:
-
Regions of interest
- SI:
-
Signal intensity
- SLEEK:
-
Spatial labeling with multiple inversion pulses
References
Messerli FH, Williams B, Ritz E (2007) Essential hypertension. Lancet 370:591–603
Hamrahian SM, Falkner B (2017) Hypertension in chronic kidney disease. Adv Exp Med Biol 956:307–325
Shemesh O, Golbetz H, Kriss JP, Myers BD (1985) Limitations of creatinine as a filtration marker in glomerulopathic patients. Kidney Int 28:830–838
Eikefjord E, Andersen E, Hodneland E et al (2017) Dynamic contrast-enhanced MRI measurement of renal function in healthy participants. Acta Radiol 58:748–757
Slanina M, Zizka J, Klzo L, Lojík M (2010) Contrast-enhanced MR angiography utilizing parallel acquisition techniques in renal artery stenosis detection. Eur J Radiol 75:e46–e50
Heverhagen JT, Krombach GA, Gizewski E (2014) Application of extracellular gadolinium-based MRI contrast agents and the risk of nephrogenic systemic fibrosis. Rofo 186:661–669
Otsuka T, Kaneko Y, Sato Y et al (2018) Kidney morphological parameters measured using noncontrast-enhanced steady-state free precession MRI with spatially selective inversion recovery pulse correlate with eGFR in patients with advanced CKD. Clin Exp Nephrol 22:45–54
Noda Y, Ito K, Kanki A et al (2015) Measurement of renal cortical thickness using noncontrast-enhanced steady-state free precession MRI with spatially selective inversion recovery pulse: association with renal function. J Magn Reson Imaging 41:1615–1621
Li Q, Wang D, Zhu X, Shen K, Xu F, Chen Y (2018) Combination of renal apparent diffusion coefficient and renal parenchymal volume for better assessment of split renal function in chronic kidney disease. Eur J Radiol 108:194–200
Li Q, Li J, Zhang L, Chen Y, Zhang M, Yan F (2014) Diffusion-weighted imaging in assessing renal pathology of chronic kidney disease: a preliminary clinical study. Eur J Radiol 83:756–762
Ding J, Chen J, Jiang Z, Zhou H, Di J, Xing W (2016) Assessment of renal dysfunction with diffusion-weighted imaging: comparing intra-voxel incoherent motion (IVIM) with a mono-exponential model. Acta Radiol 57:507–512
Takahashi T, Wang F, Quarles CC (2015) Current MRI techniques for the assessment of renal disease. Curr Opin Nephrol Hypertens 24:217–223
Thoeny HC, De Keyzer F, Oyen RH, Peeters RR (2005) Diffusion-weighted MR imaging of kidneys in healthy volunteers and patients with parenchymal diseases: initial experience. Radiology 235:911–917
Kanki A, Ito K, Tamada T et al (2013) Corticomedullary differentiation of the kidney: evaluation with noncontrast-enhanced steady-state free precession (SSFP) MRI with time-spatial labeling inversion pulse (time-SLIP). J Magn Reson Imaging 37:1178–1181
Kanki A, Ito K, Yamamoto A et al (2016) Evaluation of renal cortical thickness by non-contrast-enhanced MR imaging with spatially selective IR pulses: comparison between cirrhotic and non-cirrhotic patients. Br J Radiol. https://doi.org/10.1259/bjr.20150803:20150803
Pei Y, Shen H, Li J et al (2012) Evaluation of renal artery in hypertensive patients by unenhanced MR angiography using spatial labeling with multiple inversion pulses sequence and by CT angiography. AJR Am J Roentgenol 199:1142–1148
Tang H, Wang Z, Wang L et al (2014) Depiction of transplant renal vascular anatomy and complications: unenhanced MR angiography by using spatial labeling with multiple inversion pulses. Radiology 271:879–887
Wang HY, Wang J, Tang YH, Ye HY, Ma L (2015) Coronal diffusion-weighted magnetic resonance imaging of the kidney: agreement with axial diffusion-weighted magnetic imaging in terms of apparent diffusion coefficient values. Chin Med J (Engl) 128:499–503
Guo X, Gong Y, Wu Z, Yan F, Ding X, Xu X (2020) Renal artery assessment with non-enhanced MR angiography versus digital subtraction angiography: comparison between 1.5 and 3.0 T. Eur Radiol 30:1747–1754
Xu X, Lin X, Huang J et al (2017) The capability of inflow inversion recovery magnetic resonance compared to contrast-enhanced magnetic resonance in renal artery angiography. Abdom Radiol (NY) 42:2479–2487
Zhang LJ, Peng J, Wen J et al (2018) Non-contrast-enhanced magnetic resonance angiography: a reliable clinical tool for evaluating transplant renal artery stenosis. Eur Radiol 28:4195–4204
Xu Y, Wang X, Jiang X (2007) Relationship between the renal apparent diffusion coefficient and glomerular filtration rate: preliminary experience. J Magn Reson Imaging 26:678–681
Yalçin-Şafak K, Ayyildiz M, Ünel SY, Umarusman-Tanju N, Akça A, Baysal T (2016) The relationship of ADC values of renal parenchyma with CKD stage and serum creatinine levels. Eur J Radiol Open 3:8–11
de Bazelaire CMJ, Duhamel GD, Rofsky NM, Alsop DC (2004) MR imaging relaxation times of abdominal and pelvic tissues measured in vivo at 3.0 T: preliminary results. Radiology 230:652–659
Garcia DM, Duhamel G, Alsop DC (2005) Efficiency of inversion pulses for background suppressed arterial spin labeling. Magn Reson Med 54:366–372
Denic A, Mathew J, Nagineni VV et al (2018) Clinical and pathology findings associate consistently with larger glomerular volume. J Am Soc Nephrol 29:1960–1969
Seif M, Eisenberger U, Binser T et al (2016) Renal blood oxygenation level-dependent imaging in longitudinal follow-up of donated and remaining kidneys. Radiology 279:795–804
Graham-Brown MP, Singh A, Wormleighton J et al (2019) Association between native T1 mapping of the kidney and renal fibrosis in patients with IgA nephropathy. BMC Nephrol 20:256–256
Franke M, Baeßler B, Vechtel J et al (2017) Magnetic resonance T2 mapping and diffusion-weighted imaging for early detection of cystogenesis and response to therapy in a mouse model of polycystic kidney disease. Kidney Int 92:1544–1554
Bane O, Hectors SJ, Gordic S et al (2020) Multiparametric magnetic resonance imaging shows promising results to assess renal transplant dysfunction with fibrosis. Kidney Int 97:414–420
Acknowledgments
We thank Yaqi Shen, Fangqin Tan, and Jie Zhang, whose important contributions to this study were indispensable to its success.
Funding
This study has received funding by the National Natural Science Foundation of China (Grant No. 81771801 and No. 81571642).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Zhen Li.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Written informed consent was obtained from all subjects (patients) in this study.
Methodology
• retrospective
• diagnostic and prognostic study
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liang, P., Xu, C., Tripathi, P. et al. One-stop assessment of renal function and renal artery in hypertensive patients with suspected renal dysfunction: non-enhanced MRI using spatial labeling with multiple inversion pulses. Eur Radiol 31, 94–103 (2021). https://doi.org/10.1007/s00330-020-07088-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-020-07088-x