Abstract
Objective
To identify the white matter (WM) impairments of the antiretroviral therapy (ART)-naïve HIV patients by conducting a multivariate pattern analysis (MVPA) of Diffusion Tensor Imaging (DTI) data
Methods
We enrolled 33 ART-naïve HIV patients and 32 Normal controls in the current study. Firstly, the DTI metrics in whole brain WM tracts were extracted for each subject and feed into the Least Absolute Shrinkage and Selection Operators procedure (LASSO)-Logistic regression model to identify the impaired WM tracts. Then, Support Vector Machines (SVM) model was constructed based on the DTI metrics in the impaired WM tracts to make HIV-control group classification. Pearson correlations between the WM impairments and HIV clinical statics were also investigated.
Results
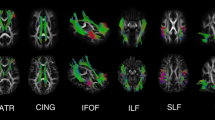
Extensive HIV-related impairments were observed in the WM tracts associated with motor function, the corpus callosum (CC) and the frontal WM. With leave-one-out cross validation, accuracy of 83.08% (P=0.002) and the area under the Receiver Operating Characteristic curve of 0.9110 were obtained in the SVM classification model. The impairments of the CC were significantly correlated with the HIV clinic statics.
Conclusion
The MVPA was sensitive to detect the HIV-related WM changes. Our findings indicated that the MVPA had considerable potential in exploring the HIV-related WM impairments.
Key points
• WM impairments along motor pathway were detected among the ART-naïve HIV patients
• Prominent HIV-related WM impairments were observed in CC and frontal WM
• The impairments of CC were significantly related to the HIV clinic statics
• The CC might be susceptible to immune dysfunction and HIV replication
• Multivariate pattern analysis had potential for studying the HIV-related white matter impairments



Similar content being viewed by others
Abbreviations
- ART:
-
Antiretroviral Therapy
- MVPA:
-
Multivariate Pattern Analysis
- DTI:
-
Diffusion tensor Imaging
- WM:
-
White Matter
- FA:
-
Fractional Anisotropy
- MD:
-
Mean Diffusivity
- AD:
-
Axial Diffusivity
- RD:
-
Radial Diffusivity
- ROI:
-
Regions of Interests
- LASSO:
-
The Least Absolute Shrinkage and Selection Operators procedure
- SVM:
-
Support Vector Machines
- ACC:
-
Accuracy
- ROC:
-
Receiver Operating Characteristic
- AUC:
-
Area under the ROC curve
- LOOCV:
-
Leave one out cross-validation
- CC:
-
Corpus Callosum
References
Ellis R, Langford D, Masliah E (2007) HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci 8:33–44
Vassallo M, Durant J, Biscay V et al (2014) Can high central nervous system penetrating antiretroviral regimens protect against the onset of HIV-associated neurocognitive disorders? Aids 28:493–501
Ances BM, Hammoud DA (2014) Neuroimaging of HIV-associated neurocognitive disorders (HAND). Curr Opin HIV AIDS 9:545–551
Clifford DB, Ances BM (2013) HIV-associated neurocognitive disorder. Lancet Infect Dis 13:976–986
Nightingale S, Winston A, Letendre S et al (2014) Controversies in HIV-associated neurocognitive disorders. Lancet Neurol 13:1139–1151
Thompson PM, Jahanshad N (2015) Novel Neuroimaging Methods to Understand How HIV Affects the Brain. Curr HIV/AIDS Rep 12:289–298
Rahimian P, He JJ (2016) HIV/neuroAIDS biomarkers. Prog Neurobiol. doi:10.1016/j.pneurobio.2016.04.003
Basser PJ, Mattiello J, LeBihan D (1994) MR diffusion tensor spectroscopy and imaging. Biophys J 66:259–267
Wright PW, Heaps JM, Shimony JS, Thomas JB, Ances BM (2012) The effects of HIV and combination antiretroviral therapy on white matter integrity. Aids 26:1501–1508
Pfefferbaum A, Rosenbloom MJ, Adalsteinsson E, Sullivan EV (2007) Diffusion tensor imaging with quantitative fibre tracking in HIV infection and alcoholism comorbidity: synergistic white matter damage. Brain 130:48–64
Pfefferbaum A, Rosenbloom MJ, Rohlfing T, Kemper CA, Deresinski S, Sullivan EV (2009) Frontostriatal fiber bundle compromise in HIV infection without dementia. Aids 23:1977–1985
Chen Y, An H, Zhu H et al (2009) White matter abnormalities revealed by diffusion tensor imaging in non-demented and demented HIV+ patients. Neuroimage 47:1154–1162
Wang B, Liu Z, Liu J, Tang Z, Li H, Tian J (2016) Gray and white matter alterations in early HIV-infected patients: Combined voxel-based morphometry and tract-based spatial statistics. J Magn Reson Imaging 43:1474–1483
Wright PW, Vaida FF, Fernandez RJ et al (2015) Cerebral white matter integrity during primary HIV infection. Aids 29:433–442
Hoare J, Fouche JP, Phillips N et al (2015) White matter micro-structural changes in ART-naive and ART-treated children and adolescents infected with HIV in South Africa. Aids 29:1793–1801
Correa DG, Zimmermann N, Doring TM et al (2015) Diffusion tensor MR imaging of white matter integrity in HIV-positive patients with planning deficit. Neuroradiology 57:475–482
Tran LT, Roos A, Fouche JP et al (2016) White Matter Microstructural Integrity and Neurobehavioral Outcome of HIV-Exposed Uninfected Neonates. Medicine (Baltimore) 95, e2577
Ackermann C, Andronikou S, Saleh M et al (2016) Early Antiretroviral Therapy in HIV-Infected Children Is Associated with Diffuse White Matter Structural Abnormality and Corpus Callosum Sparing. Am J Neuroradiol
Masters MC, Ances BM (2014) Role of neuroimaging in HIV-associated neurocognitive disorders. Semin Neurol 34:89–102
Hu X, Liu Q, Li B et al (2016) Multivariate pattern analysis of obsessive–compulsive disorder using structural neuroanatomy. Eur Neuropsychopharmacol 26:246–254
Bertocci MA, Bebko G, Versace A et al (2016) Predicting clinical outcome from reward circuitry function and white matter structure in behaviorally and emotionally dysregulated youth. Mol Psychiatry 21:1194–1201
Li F, Huang X, Tang W et al (2014) Multivariate pattern analysis of DTI reveals differential white matter in individuals with obsessive-compulsive disorder. Hum Brain Mapp 35:2643–2651
Bron EE, Smits M, Papma JM et al (2016) Multiparametric computer-aided differential diagnosis of Alzheimer's disease and frontotemporal dementia using structural and advanced MRI. Eur Radiol. doi:10.1007/s00330-016-4691-x
Kamagata K, Hatano T, Okuzumi A et al (2016) Neurite orientation dispersion and density imaging in the substantia nigra in idiopathic Parkinson disease. Eur Radiol 26:2567–2577
Uban KA, Herting MM, Williams PL et al (2015) White matter microstructure among youth with perinatally acquired HIV is associated with disease severity. Aids 29:1035–1044
Nir TM, Jahanshad N, Busovaca E et al (2014) Mapping white matter integrity in elderly people with HIV. Hum Brain Mapp 35:975–992
Tibshirani R (1996) Regression shrinkage and selection via the Lasso. J R Stat Soc Ser B Methodol 58:267–288
Bunea F, She Y, Ombao H, Gongvatana A, Devlin K, Cohen R (2011) Penalized least squares regression methods and applications to neuroimaging. Neuroimage 55:1519–1527
Shah A, Gangwani MR, Chaudhari NS, Glazyrin A, Bhat HK, Kumar A (2016) Neurotoxicity in the Post-HAART Era: Caution for the Antiretroviral Therapeutics. Neurotox Res 30:677–697
Fang P, An J, Zeng LL et al (2015) Multivariate pattern analysis reveals anatomical connectivity differences between the left and right mesial temporal lobe epilepsy. Neuroimage Clin 7:555–561
Grana M, Termenon M, Savio A et al (2011) Computer aided diagnosis system for Alzheimer disease using brain diffusion tensor imaging features selected by Pearson's correlation. Neurosci Lett 502:225–229
Mori S, Oishi K, Jiang H et al (2008) Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage 40:570–582
Smith SM, Jenkinson M, Woolrich MW et al (2004) Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23:S208–S219
Smith SM (2002) Fast robust automated brain extraction. Hum Brain Mapp 17:143–155
Woolrich MW, Jbabdi S, Patenaude B et al (2009) Bayesian analysis of neuroimaging data in FSL. Neuroimage 45:S173–S186
Cui Z, Zhong S, Xu P, He Y, Gong G (2013) PANDA: a pipeline toolbox for analyzing brain diffusion images. Front Hum Neurosci 7:42
Tang Z, Dong E, Liu J et al (2016) Longitudinal assessment of fractional anisotropy alterations caused by simian immunodeficiency virus infection: a preliminary diffusion tensor imaging study. J Neurovirol 22:231–239
Gunbey HP, Bilgici MC, Aslan K et al (2016) Structural brain alterations of Down's syndrome in early childhood evaluation by DTI and volumetric analyses. Eur Radiol. doi:10.1007/s00330-016-4626-6
Ryu CW, Park MS, Byun JY, Jahng GH, Park S (2016) White matter integrity associated with clinical symptoms in tinnitus patients: A tract-based spatial statistics study. Eur Radiol 26:2223–2232
Wilting J, Rolfsnes HO, Zimmermann H et al (2016) Structural correlates for fatigue in early relapsing remitting multiple sclerosis. Eur Radiol 26:515–523
Friedman J, Hastie T, Tibshirani R (2010) Regularization Paths for Generalized Linear Models via Coordinate Descent. J Stat Softw 33:1–22
Ojala M, Garriga GC (2010) Permutation Tests for Studying Classifier Performance. J Mach Learn Res 11:1833–1863
Tian L, Ma L, Wang L (2016) Alterations of functional connectivities from early to middle adulthood: Clues from multivariate pattern analysis of resting-state fMRI data. Neuroimage 129:389–400
Zeng LL, Shen H, Liu L et al (2012) Identifying major depression using whole-brain functional connectivity: a multivariate pattern analysis. Brain 135:1498–1507
Bernard C, Dilharreguy B, Allard M et al (2013) Muscular weakness in individuals with HIV associated with a disorganization of the cortico-spinal tract: a multi-modal MRI investigation. Plos One 8, e66810
DeVaughn S, Muller-Oehring EM, Markey B, Bronte-Stewart HM, Schulte T (2015) Aging with HIV-1 Infection: Motor Functions, Cognition, and Attention--A Comparison with Parkinson's Disease. Neuropsychol Rev 25:424–438
Wilson TW, Heinrichs-Graham E, Robertson KR et al (2013) Functional brain abnormalities during finger-tapping in HIV-infected older adults: a magnetoencephalography study. J Neuroimmune Pharmacol 8:965–974
Tang VM, Lang DJ, Giesbrecht CJ et al (2015) White matter deficits assessed by diffusion tensor imaging and cognitive dysfunction in psychostimulant users with comorbid human immunodeficiency virus infection. BMC Res Notes 8:515
Zhu T, Zhong J, Hu R et al (2013) Patterns of white matter injury in HIV infection after partial immune reconstitution: a DTI tract-based spatial statistics study. J Neurovirol 19:10–23
Stubbe-Drger B, Deppe M, Mohammadi S et al (2012) Early microstructural white matter changes in patients with HIV: a diffusion tensor imaging study. BMC Neurol 12:23
Heaps-Woodruff JM, Wright PW, Ances BM, Clifford D, Paul RH (2016) The impact of human immune deficiency virus and hepatitis C coinfection on white matter microstructural integrity. J Neurovirol 22:389–399
Kelly SG, Taiwo BO, Wu Y et al (2014) Early suppressive antiretroviral therapy in HIV infection is associated with measurable changes in the corpus callosum. J Neurovirol 20:514–520
Leite SC, Correa DG, Doring TM et al (2013) Diffusion tensor MRI evaluation of the corona radiata, cingulate gyri, and corpus callosum in HIV patients. J Magn Reson Imaging 38:1488–1493
Kamat R, Brown GG, Bolden K et al (2014) Apathy is associated with white matter abnormalities in anterior, medial brain regions in persons with HIV infection. J Clin Exp Neuropsychol 36:854–866
Hoare J, Fouche JP, Phillips N et al (2015) Clinical associations of white matter damage in cART-treated HIV-positive children in South Africa. J Neurovirol 21:120–128
Sousa AE, Carneiro J, Meier-Schellersheim M, Grossman Z, Victorino RMM (2002) CD4 T cell depletion is linked directly to immune activation in the pathogenesis of HIV-1 and HIV-2 but only indirectly to the viral load. J Immunol 169:3400–3406
Sainz T, Serrano-Villar S, Diaz L et al (2013) The CD4/CD8 ratio as a marker T-cell activation, senescence and activation/exhaustion in treated HIV-infected children and young adults. Aids 27:1513–1516
Sun SW, Liang HF, Trinkaus K, Cross AH, Armstrong RC, Song SK (2006) Noninvasive detection of cuprizone induced axonal damage and demyelination in the mouse corpus callosum. Magn Reson Med 55:302–308
Acknowledgements
This paper was supported by the National Natural Science Foundation of China under Grant No. 81671848, 81501549, 81527805, 81371635, 81227901, 61231004, 81571634, the Science and Technology Service Network Initiative Program of Chinese Academy of Science under Grant NO. KFJ-SW-STS-160, the Strategic Priority Research Program from the Chinese Academy of Sciences under Grant NO. XDB02060010, the Beijing Municipal Science & Technology Commission No. Z161100002616022, the Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding under Grant NO. ZYLX201511, and the Beijing Municipal Administration of Hospitals Incubating Program under Grant NO. PX2016036. The study was approved by the Ethics Committee of the Beijing YouAn Hospital, Capital Medical University. All the subjects had provided written informed consent after a detailed explanation of this study. The authors would like to express their deep appreciation to all anonymous reviewers for their kind comments.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Jie Tian.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Funding
This paper is supported by the National Natural Science Foundation of China under Grant No. 81671848, 81501549, 81527805, 81371635, 81227901, 61231004, 81571634, Science and Technology Service Network Initiative Program of Chinese Academy of Science under Grant NO. KFJ-SW-STS-160, the Strategic Priority Research Program from Chinese Academy of Sciences under Grant NO. XDB02060010, the Beijing Municipal Science & Technology Commission No. Z161100002616022, the Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding under Grant NO. ZYLX201511, and the Beijing Municipal Administration of Hospitals Incubating Program under Grant NO. PX2016036.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects (patients) in this study.
Ethical approval
The study was approved by the Ethics Committee of the Beijing YouAn Hospital, Capital Medical University.
Methodology
• retrospective
• cross sectional study
• performed at one institution
Rights and permissions
About this article
Cite this article
Tang, Z., Liu, Z., Li, R. et al. Identifying the white matter impairments among ART-naïve HIV patients: a multivariate pattern analysis of DTI data. Eur Radiol 27, 4153–4162 (2017). https://doi.org/10.1007/s00330-017-4820-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-017-4820-1