Abstract
Objectives
Screening of living kidney donors may require scintigraphy to split glomerular filtration rate (GFR). To determine the usefulness of computed tomography (CT) to split GFR, we compared scintigraphy-split GFR to CT-split GFR. We evaluated CT-split GFR as a screening test to detect scintigraphy-split GFR lower than 40 mL/min/1.73 m2/kidney.
Methods
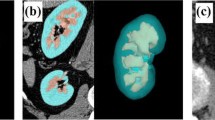
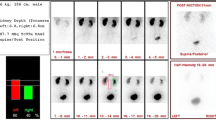
This was a monocentric retrospective study on 346 potential living donors who had GFR measurement, renal scintigraphy, and CT. We predicted GFR for each kidney by splitting GFR using the following formula: Volume-split GFR for a given kidney = measured GFR*[volume of this kidney/(volume of this kidney + volume of the opposite kidney)]. The same formula was used for length-split GFR. We compared length- and volume-split GFR to scintigraphy-split GFR at donation and with a 4-year follow-up.
Results
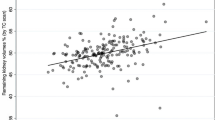
A better correlation was observed between length-split GFR and scintigraphy-split GFR (r = 0.92) than between volume-split GFR and scintigraphy-split GFR (r = 0.89). A length-split GFR threshold of 45 mL/min/1.73 m2/kidney had a sensitivity of 100 % and a specificity of 75 % to detect scintigraphy-split GFR less than 40 mL/min/1.73 m2/kidney. Both techniques with their respective thresholds detected living donors with similar eGFR evolution during follow-up.
Conclusion
Length-split GFR can be used to detect patients requiring scintigraphy.
Key points
• Excellent correlation between kidney length and scintigraphy predicted GFR
• Kidney length screening detects all donors with GFR lower than 40 mL/min/1.73 m 2
• Kidney length screening can replace scintigraphy screening.





Similar content being viewed by others
Abbreviations
- AUC:
-
area under the curve
- CT:
-
computed tomography
- Cr-EDTA:
-
51Cr-ethylene-diamine tetra acetic acid
- Tc-DTPA:
-
99mTc-diethylene-triamine penta acetic acid
- GFR:
-
glomerular filtration rate
- MDRD:
-
modification of diet in renal disease
- ROC:
-
receiver operating characteristics
- ROI:
-
region of interest
- SRF:
-
split renal function
- SRV:
-
split renal volume
- OLS:
-
ordinary least squares
References
Hariharan S, Johnson CP, Bresnahan BA, Taranto SE, McIntosh MJ, Stablein D (2000) Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med 342:605–612
Legendre C, Canaud G, Martinez F (2014) Factors influencing long-term outcome after kidney transplantation. Transpl Int 27:19–27
Garg AX, Muirhead N, Knoll G, Yang RC, Prasad GV, Thiessen-Philbrook H et al (2006) (Donor Nephrectomy Outcomes Research (DONOR) Network). Proteinuria and reduced kidney function in living kidney donors: A systematic review, meta-analysis, and meta-regression. Kidney Int 70(10):1801–1810
Ibrahim HN, Foley R, Tan L, Rogers T, Bailey RF, Guo H et al (2009) Long-term consequences of kidney donation. N Engl J Med 360(5):459–469
Mjoen G, Hallan S, Hartmann A, Foss A, Midtvedt A, Oyen O et al (2014) Long-term risks for kidney donors. Kidney Int 86:162–167
Muzaale AD, Massie AB, Wang MC, Montgomery RA, McBride MA, Wainright JL et al (2014) Risk of end-stage renal disease following live kidney donation. JAMA 311:579–586
Yakoubi R, Autorino R, Kassab A, Long JA, Haber GP, Kaouk JH (2013) Does preserved kidney volume predict 1 year donor renal function after laparoscopic living donor nephrectomy? Int J Urol 20(9):931–934
Tanriover B, Fernandez S, Campenot ES, Newhouse JH, Oyfe I, Mohan P, Sandikci B, Radhakrishnan J, Wexler JJ, Carroll MA, Sharif S, Cohen DJ, Ratner LE, Hardy MA (2015) Live Donor Renal Anatomic Asymmetry and Posttransplant Renal Function. Transplantation 99(8):e66–e74
Moore DR, Serur D, Rudow DL, Rodrigue JR, Hays R, Cooper M (2015) Living Donor Kidney Transplantation: Improving Efficiencies in Live Kidney Donor Evaluation-Recommendations from a Consensus Conference. Clin J Am Soc Nephrol 10(9):1678–1686
Diez A, Powelson J, Sundaram CP, Taber TE, Mujtaba MA, Yaqub MS et al (2014) Correlation between CT-based measured renal volumes and nuclear-renography-based split renal function in living kidney donors. Clinical diagnostic utility and practice patterns. Clin Transplant 28(6):675–682
Patankar K, Su-Tong Low R, Blakeway D (2014) Ferrari P Comparison of computer tomographic volumetry versus nuclear split renal function to determine residual renal function after living kidney donation. Acta Radiol 55(6):753–760
Halleck F, Diederichs G, Koehlitz T, Slowinski T, Engelken F, Liefeldt L et al (2013) Volume matters: CT-based renal cortex volume measurement in the evaluation of living kidney donors. Transplantation International 26:1208–1216
Yokoyama N, Ishimura T (2015) Usefulness of three-dimensional computerized tomographic volumetry for determining split renal function in donors for living-related kidney transplantation. Transplant Proc 47(3):588–590
Breau RH, Clark E, Bruner B, Cervini P, Atwell T, Knoll G et al (2013) A simple method to estimate renal volume from computed tomography. Can Urol Assoc J 7(5-6):189–192
Summerlin AL, Lockhart ME, Strang AM, Kolettis PN, Fineberg NS, Smith JK (2008) Determination of split renal function by 3D reconstruction of CT angiograms: a comparison with gamma camera renography. AJR Am J Roentgenol 191:1552–1558
Kato F, Kamishima T, Morita K, Muto NS, Okamoto S, Omatsu T et al (2011) Rapid estimation of split renal function in kidney donors using software developed for computed tomographic renal volumetry. Eur J Radiol 79:15–20
Soga S, Britz-Cunningham S, Kumamaru KK, Malek SK, Tullius SG, Rybicki FJ (2012) Comprehensive comparative study of computed tomography-based estimates of split renal function for potential renal donors: modified ellipsoid method and other CT-based methods. J Comput Assist Tomogr 36:323–329
Piepsz A, Kinthaert J, Tondeur M, Ham HR (1996) The robustness of the Patlak-Rutland slope for the determination of split renal function. Nucl Med Commun 17(9):817–821
Delmonico FL: A report of the Amsterdam forum on thecare of the live kidney donor: data and medical guidelines.Transplantation 79[Suppl]: S53–S66, 2005
Bia MJ, Ramos EL, Danovitch GM, Gaston RS, Harmon WE, Leichtman AB et al (1995) Evaluation of living renal donors. The current practice of US transplant centers. Transplantation 60:322–327
Gabolde M, Herve C, Moulin AM (2001) Evaluation, selection, and follow-up of live kidney donors: A review of current practice in French renal transplant centres. Nephrol Dial Transplant 16:2048–2052
Rule AD, Gussak HM, Pond GR, Bergstralh EJ, Stegall MD, Cosio FG et al (2004) Measured and estimated GFR in healthy potential kidney donors. Am J Kidney Dis 43:112–119
Davis CL, Delmonico FL (2005) Living-Donor Kidney Transplantation: A Review of the Current Practices for the Live Donor. J Am Soc Nephrol 16:2098–2110
Acknowledgments
The scientific guarantor of this publication is Marie Courbebaisse. François Gaillard thanks Ecole de l'INSERM-Liliane Bettencourt. The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding.
No complex statistical methods were necessary for this paper. Institutional Review Board approval was obtained, REF2013-11-10. Written informed consent was waived by the Institutional Review Board. Methodology: retrospective, diagnostic or prognostic study, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Additional information
Christophe Legendre and Marie Courbebaisse contributed equally to this work.
Rights and permissions
About this article
Cite this article
Gaillard, F., Pavlov, P., Tissier, AM. et al. Use of computed tomography assessed kidney length to predict split renal GFR in living kidney donors. Eur Radiol 27, 651–659 (2017). https://doi.org/10.1007/s00330-016-4410-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-016-4410-7