Abstract
Objectives
The aim was to determine the proton MR (1H-MR) spectra of normal adult testes and variations with age.
Methods
Forty-one MR spectra of normal testes, including 16 testes from men aged 20–39 years (group I) and 25 testes from men aged 40–69 years (group II), were analyzed. A single-voxel point-resolved spectroscopy sequence (PRESS), with TR/TE: 2000/25 ms was used. The volume of interest was placed to include the majority of normal testicular parenchyma. Association between normalized metabolite concentrations, defined as ratios of the calculated metabolite concentrations relative to creatine concentration, and age was assessed.
Results
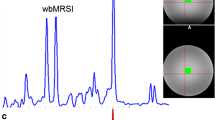
Quantified metabolites of the spectra were choline (Cho), creatine (Cr), myo-inositol (mI), scyllo-inositol, taurine, lactate, GLx compound, glucose, lipids, and macromolecules resonating at 0.9 ppm (LM09), around 20 ppm (LM20), and at 13 ppm (LM13). Most prominent peaks were Cho, Cr, mI, and lipids. A weak negative correlation between mI and age (P = 0.015) was observed. Higher normalized concentrations of Cho (P = 0.03), mI (P = 0.08), and LM13 (P = 0.05) were found in group I than in group II.
Conclusions
1H-MR spectra of a normal adult testis showed several metabolite peaks. A decrease of levels of Cho, mI, and LM13 was observed with advancing age.
Key Points
• Single-voxel PRESS MRS of a normal testis is feasible.
• 1H-MR spectra of a normal testis showed several metabolite peaks.
• Most prominent peaks were Cho, Cr, mI, and lipids.
• A decrease of Cho, mI, and LM13 was seen with advancing age.


Similar content being viewed by others
Abbreviations
- 1H-MRS:
-
proton magnetic resonance spectroscopy
- PRESS:
-
point-resolved spectroscopy sequence
- VOI:
-
volume of interest
- FOV:
-
field of view
- HRMAS:
-
high resolution magic angle spinning
- Ppm:
-
parts per million
- Cho:
-
choline
- Cr:
-
creatine
- Glc:
-
glucose
- mI:
-
myo-inositol
- Lac:
-
lactate
- Scyllo:
-
scyllo-inositol
- Tau:
-
taurine
- Glx:
-
glutamine and glutamate
- LM09 LM13, LM20:
-
lipids and macromolecules resonating at 0.9, 1.3 ppm, and 2.0 ppm, respectively
- NOA:
-
non-obstructive azoospermia
- SCO:
-
Sertoli-cell only
- MA:
-
maturation arrest
References
Dogra VS, Gottlieb RH, Oka M, Rubens DJ (2003) Sonography of the scrotum. Radiology 227:18–36
Tsili AC, Giannakis D, Sylakos A, Ntorkou A, Sofikitis N, Argyropoulou MI (2014) MR imaging of scrotum. Magn Reson Imaging Clin N Am 22:217–238
Aganovic L, Cassidy F (2012) Imaging of the scrotum. Radiol Clin North Am 50:1145–1165
Dieckmann KP, Frey U, Lock G (2013) Contemporary diagnostic work-up of testicular germ cell tumours. Nat Rev Urol 10:703–712
Bhatt S, Jafri SZ, Wasserman N, Dogra VS (2011) Imaging of non-neoplastic intratesticular masses. Diagn Interv Radiol 17:52–63
Woldrich JM, Im RD, Hughes-Cassidy FM, Aganovic L, Sakamoto K (2013) Magnetic resonance imaging for intratesticular and extratesticular scrotal lesions. Can J Urol 20:6855–6859
Valentino M, Bertolotto M, Derchi L et al (2011) Role of contrast enhanced ultrasound in acute scrotal diseases. Eur Radiol 21:1831–1840
Bertolotto M, Derchi LE, Sidhu PS et al (2011) Acute segmental testicular infarction at contrast-enhanced ultrasound: early features and changes during follow-up. AJR Am J Roentgenol 196:834–841
Tsili AC, Argyropoulou MI, Astrakas LG et al (2013) Dynamic contrast-enhanced subtraction MRI for characterizing intratesticular mass lesions. AJR Am J Roentgenol 200:578–585
Watanabe Y, Dohke M, Ohkudo K et al (2000) Scrotal disorders: evaluation of testicular enhancement patterns at dynamic contrast-enhanced subtraction MR imaging. Radiology 217:219–227
Reinges MHT, Kaiser WA, Miersch WD, Vogel J, Reiser M (1995) Dynamic MRI of benign and malignant testicular lesions: preliminary observations. Eur Radiol 5:615–622
Tsili AC, Argyropoulou MI, Giannakis D, Tsampalas S, Sofikitis N, Tsampoulas K (2012) Diffusion-weighted MR imaging of normal and abnormal scrotum: preliminary results. Asian J Androl 14:649–654
Kato T, Kojima Y, Kamisawa H et al (2011) Findings of fat-suppressed T2-weighted and diffusion-weighted magnetic resonance imaging in the diagnosis of non-palpable testes. BJU Int 107:290–294
Maki D, Watanabe Y, Nagayama M et al (2011) Diffusion-weighted magnetic resonance imaging in the detection of testicular torsion: feasibility study. J Magn Reson Imaging 34:1137–1142
Figueiras RG, Gonzalez SB, Cobas C, et al (2013) Searching for fingerprint images: Magnetic resonance spectroscopy in oncology. 99th Scientific Assembly and Annual Meeting, Chicago. Available via http://rsna2013.rsna.org/program/details/?publicid=CL-MIE4115
Di Costanzo A, Scarabino T, Trojsi F et al (2008) Proton MR spectroscopy of cerebral gliomas at 3T: spatial heterogeneity, and tumour grade and extent. Eur Radiol 18:1727–1735
Fischbach F, Schirmer T, Thormann M, Freund T, Ricke J, Bruhn H (2008) Quantitative proton magnetic resonance spectroscopy of the normal liver and malignant hepatic lesions at 3.0 Tesla. Eur Radiol 11:2549–2558
Machann J, Stefan N, Schick F (2008) 1H MR spectroscopy of skeletal muscle, liver and bone marrow. Eur J Radiol 67:275–284
Dorrius MD, Pijnappel RM, van der Weide Jansen MC et al (2012) The added value of quantitative multi-voxel MR spectroscopy in breast magnetic resonance imaging. Eur Radiol 22:915–922
Lichy MP, Pintaske J, Kottke R et al (2005) 3D proton MR spectroscopic imaging of prostate cancer using a standard spine coil at 1.5 T in clinical routine: a feasibility study. Eur Radiol 15:653–660
Umbehr M, Bachmann LM, Held U et al (2009) Combined magnetic resonance imaging and magnetic resonance spectroscopy imaging in the diagnosis of prostate cancer: a systematic review and meta-analysis. Eur Urol 55:575–590
Russo F, Mazzetti S, Grignani G et al (2012) In vivo characterisation of soft tissue tumours by 1.5-T proton MR spectroscopy. Eur Radiol 22:1131–1139
Zhang J, Cai S, Li C et al (2014) Can magnetic resonance spectroscopy differentiate endometrial cancer? Eur Radiol 24:2552–2560
Takeuchi M, Matsuzaki K, Harada M (2011) Differentiation of benign and malignant uterine corpus tumors by using proton MR spectroscopy at 3T: preliminary study. Eur Radiol 21:850–856
Takeuchi M, Matsuzaki K, Harada M (2013) Preliminary observations and clinical value of lipid peak in high-grade uterine sarcomas using in vivo proton MR spectroscopy. Eur Radiol 23:2358–2363
Tzika AA, Vigneron DB, Hricak H, Moseley ME, James TL, Kogan BA (1989) P-31 MR spectroscopy in assessing testicular torsion: rat model. Radiology 172:753–757
Srinivas M, Degaonkar M, Chandrasekharam VV et al (2002) Potential of MRI and 31P MRS in the evaluation of experimental testicular trauma. Urology 59:969–972
van der Grond J, Laven JS, van Echteld CJ et al (1992) The progression of spermatogenesis in the developing rat testis followed by 31P MR spectroscopy. Magn Reson Med 23:264–274
Sasagawa I, Tateno T, Yazawa H, Ichiyanagi O, Nakada T (1998) Assessment of testicular function in experimental varicocele rats by phophorus-31 magnetic resonance spectroscopy. Urol Res 26:407–410
Sasagawa I, Nakada T, Kubota Y, Ishigooka M, Uchida K, Doi K (1995) In vivo 31P magnetic resonance spectroscopy for evaluation of testicular function in cryptorchid rats. J Urol 154:1557–1559
Bretan PN Jr, Vigneron DB, Hricak H et al (1987) Assessment of testicular metabolic integrity with P-31 MR spectroscopy. Radiology 162:867–871
van der Grond J, Laven JS, te Velde ER, Mali WP (1991) Abnormal testicular function: potential of P-31 MR spectroscopy in diagnosis. Radiology 179:433–436
Chew WM, Hricak H, McClure RD, Wendland MF (1990) In vivo human testicular function assessed with P-31 MR spectroscopy. Radiology 177:743–747
Rohr G, Eggert-Kruse W, Kalbitzer HR (1995) NMR spectroscopy in andrology: research uses and possible clinical applications. Int J Androl 18:12–19
Kiricuta IC, Bluemm RG, Rühl J, Beyer HK (1994) 31-P MR spectroscopy and MRI of a testicular non-Hodgkin lymphoma recurrence to monitor response to irradiation. A case report. Strahlenther Onkol 170:359–364
Griffin JL, Troke J, Walker LA, Shore RF, Lindon JC, Nicholson JK (2000) The biochemical profile of rat testicular tissue as measured by magic angle spinning 1H NMR spectroscopy. FEBS Lett 486:225–229
Yamaguchi M, Mitsumori F, Watanabe H, Takaya N, Minami M (2006) In vivo localized 1H MR spectroscopy of rat testes: stimulated echo acquisition mode (STEAM) combined with short TI inversion recovery (STIR) improves the detection of metabolite signals. Magn Reson Med 55:749–754
Thomsen C, Jensen KE, Giwercman A, Kjaer L, Henriksen O, Skakkebaek NE (1987) Magnetic resonance: in vivo tissue characterization of the testes in patients with carcinoma-in-situ of the testis and healthy subjects. Int J Androl 10:191–198
Rasalkar DD, Paunipagar BK, Ng A, Lai FM, Bagaria SJ (2011) Intra-abdominal testicular seminoma in a woman with testicular feminization syndrome. Case Rep Radiol 2011:592124
Aaronson DS, Iman R, Walsh TJ, Kurhanewicz J, Turek PJ (2010) A novel application of 1H magnetic resonance spectroscopy: non-invasive identification of spermatogenesis in men with non-obstructive azoospermia. Hum Reprod 25:847–852
Firat AK, Uğraş M, Karakaş HM et al (2008) 1H magnetic resonance spectroscopy of the normal testis: preliminary findings. Magn Reson Imaging 26:215–220
Wilson M, Reynolds MG, Kauppinen RA, Arvanitis TN, Peet AC (2011) A constrained least-squares approach to the automated quantitation of in vivo 1H magnetic resonance spectroscopy data. Magn Reson Med 65:1–12
Turek PJ (2012) Male reproductive physiology. In: Wein AJ, Kavoussi LR, Novick AC, Partin AW, Peters CA (eds) Campbell-Walsh urology, ed. 10. Elsevier Inc, Philadelphia, pp 591–605
Paniagua R, Nistal M, Amat P, Rodriguez MC, Martin A (1987) Seminiferous tubule involution in elderly men. Biol Reprod 36:939–947
Kerr JB (1992) Functional cytology of the human testis. Baillieres Clin Endocrinol Metab 6:235–250
Smith LB, Walker WH (2014) The regulation of spermatogenesis by androgens. Semin Cell Dev Biol 30:2–13
Walker WH (2011) Testosterone signaling and the regulation of spermatogenesis. Spermatogenesis 1:116–120
Keber R, Rozman D, Horvat S (2013) Sterols in spermatogenesis and sperm maturation. J Lipid Res 54:20–33
Chauvin TR, Griswold MD (2004) Characterization of the expression and regulation of genes necessary for myo-inositol biosynthesis and transport in the seminiferous epithelium. Biol Reprod 70:744–751
Guan G, Dai P, Shechter I (2003) cDNA cloning and gene expression analysis of human myo-inositol 1-phosphate synthase. Arch Biochem Biophys 15:251–259
Robinson R, Fritz IB (1979) Myoinositol biosynthesis by Sertoli cells, and levels of myoinositol biosynthetic enzymes in testis and epididymis. Can J Biochem 57:962–967
Condorelli RA, La Vignera S, Bellanca S, Vicari E, Calogero AE (2012) Myoinositol: does it improve sperm mitochondrial function and sperm motility? Urology 79:1290–1295
Carlomagno G, Nordio M, Chiu TT, Unfer V (2011) Contribution of myo-inositol and melatonin to human reproduction. Eur J Obstet Gynecol Reprod Biol 159:267–272
Acknowledgments
The scientific guarantor of this publication is Dr. Athina C. Tsili.
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
The authors state that this work has not received any funding.
Loukas Astrakas kindly provided statistical advice for this manuscript and is one of the authors of this paper.
Informed consent was obtained from all participants prior to the examinations, which were previously approved by the local ethics committee.
Methodology: prospective, diagnostic or prognostic study, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsili, A.C., Astrakas, L.G., Ntorkou, A. et al. MR Spectra of Normal Adult Testes and Variations with Age: Preliminary Observations. Eur Radiol 26, 2261–2267 (2016). https://doi.org/10.1007/s00330-015-4055-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-015-4055-y