Abstract
Purpose
To compare the dose estimates and image quality of Dual Energy CT (DECT), Dual Source CT (DSCT) and 16-slice CT for coronary CT angiography (cCTA).
Methods
Sixty-eight patients were examined with 16 - slice MDCT (group 1), 68 patients with DSCT (group 2) and 68 patients using DSCT in dual energy mode (DECT group 3). CT dose index volume, dose length product, effective dose, signal-to-noise, and contrast-to-noise ratio were compared. Subjective image quality was rated by two observers, blinded to technique.
Results
The mean estimated radiation dose of all patients investigated on a 16 - slice MDCT was 12 ± 3.59 mSv, for DSCT in single energy 9.8 ± 4.77 mSv and for DECT 4.54 ± 1.87 mSv. Dose for CTA was significantly lower in group 3 compared to group 1 and 2. The image noise was significantly lower in Group 2 in comparison to group 1 and group 3. There was no significant difference in diagnostic image quality comparing DECT and DSCT.
Conclusion
cCTA shows better dose levels at both DECT and DSCT compared to 16-slice CT. Further, DECT delivers significantly less dose than regular DSCT or single source single energy cCTA while maintaining diagnostic image quality.
Similar content being viewed by others
Introduction
CT of the heart has evolved into an important clinical tool for the assessment of coronary artery disease [1, 2]. According to recent guidelines, its use is considered appropriate for symptomatic patients at intermediate risk for coronary artery disease [3, 4]. Although imaging CAD using coronary CT angiography (cCTA) is nowadays in clinical routine, the prediction of the hemodynamic significance of CAD based on anatomical data has been reason for concern.
However, recent research has demonstrated the potential use of dual-energy CT (DECT) and different tube voltage/tube current protocols for the characterization of tissue components and organ perfusion as well as for dose reduction [5–11]. In addition there is evidence that suggests the usefulness of DECT for comprehensive imaging of coronary artery disease and the evaluation of myocardial perfusion [12, 13].
Because CT represents the most important source of ionizing radiation arising from medical exposures, it is necessary to study radiation exposure that is received during coronary CT angiography in daily practice [14, 15]. The clinically acceptability of DECT of the heart is consequently directly related to radiation dose exposure in routine use compared to other cCTA techniques.
Therefore the purpose of our study was to retrospectively compare the effective dose estimates as well as the influence on image quality of DECT compared to those of 16-slice MDCT and dual source CT (DSCT) in cCTA in patients that were clinically referred to our department to rule out coronary artery disease using a standardized protocol for retrospectively ECG-gated spiral computed tomography.
Material and methods
Patient collective
The study was approved by the institutional review board. Data from 204 consecutive patients who have had clinically indicated cCTA examination were retrospectively included in this study. The indications were in concordance with current guidelines and recommendations [3]. The data analyzed retrospectively in this study, were acquired in a time frame of 2,5 years, starting in September 2006, ending in March 2009. Group 1 (n = 68) was examined on a 16-slice CT (Somatom Sensation 16, Siemens, Forchheim, Germany), Group 2 (n = 68) was examined using DSCT (Somatom Definition, Siemens, Forchheim, Germany). Group 3 (n = 68) was examined on a DSCT using dual energy mode (Somatom Definition, Siemens, Forchheim, Germany). Exclusion criteria were as follows: (a) unstable symptoms, vital signs, high heart rates (>85 bpm) and frequent extra systole; (b) creatinine level of more than 1.5 mg/dL; (c) potential pregnancy; and (d) known previous reaction to iodinated contrast material. Unstable symptoms describes a situation of unexpected chest pain usually occurring while at rest and with a discomfort that is either more severe and prolonged than that caused by typical angina or that represents the first time a person has had angina. Implanted stents, coronary bypass vessels, or valve prosthesis was not regarded as exclusion criteria.
Image acquisition
A commercially available dual-source CT system (Somatom Definition; Siemens Medical Solutions, Forchheim, Germany) was used to perform DSCT and dual-energy coronary CT angiography. The dual-source CT system is equipped with two x-ray tubes (tube A and B) and two corresponding detector arrays. These two acquisition systems are mounted perpendicularly to each other within the gantry. Depending on the number of acquisition systems being used, the CT system can be operated in different basic CT protocols: in single-source or dual-source mode. Dual-source setting can subsequently be used for dual-energy data acquisition by operating both tubes at different potentials (typically 80 kV and 140 kV) generating different x-ray spectra. Due to the x-ray spectrum of different materials, dual energy CT brings the option for material differentiation—especially for the separation of iodine—into clinical routine [7].
CT parameters were as follows: For 16 slice CT a collimation 16 × 0.75 mm, table feed of 2.8 mm/rotation, tube voltage of 120 kV, tube current time product of 500 mAs and a gantry rotation time of 420 ms was used. For patient group 2 CT was performed using DSCT: 2 × 64 × 0.6 mm collimation, 120 KV, 320 mAs/rotation and automated pitch adaptation, depending on the patient’s heart rate. The DSCT protocol for group 3 was performed using Dual Energy Mode using the following parameters: 2 × 64 × 0.6 mm collimation, 140 KV (A-Tube)/100KV (B-Tube), 100 mAs/rot (A-Tube) 165 mAs/rot (B-Tube), 330 ms rotation time, pitch automatically adapted by the DSCT system’s software depending on the patient’s heart rate. In group 2 and 3 dose modulation was used (CareDose, Siemens, Germany). In all groups patients with a heart rate above 70 bpm received up to 10 mg B-Blockers. In all patients the anatomical region examined extended from the level of the carina to just below the dome of the diaphragm (Tables 1 and 2).
Contrast media injection protocol
In group 2 and group 3 all CT examinations were performed by using nonionic, low-osmolar contrast medium injected through an 18-gauge intravenous antecubital catheter (Imeron 400; Bracco Altana Pharma, Konstanz, Germany). A test bolus approach was performed to determine the contrast agent transit time (15-mL contrast material followed by 50 mL of saline with a flow rate of 5 mL/sec). Consecutive single-level CT sections at the level of the aortic root were obtained every 2 sec during breath holding. The aortic time-resolved attenuation was then measured by using the time-attenuation evaluation program accessible on the CT system, and the time of peak attenuation was used as the delay time for the actual examination. The actual CT data acquisition was performed with injection of 50–75 mL of pure, undiluted iodinated contrast material followed by a constant volume of 50 mL of a 70%:30% saline-to-contrast medium mixture and 30 mL of pure saline with a flow rate of 5 mL/sec. The contrast medium volume for the first iodine phase of injection was individually computed according to the following formula: V = ST · 5, where V is volume in millilitres and ST is CT data acquisition time in seconds.
In group 1, each patient received 90 mL of a nonionic contrast medium (Ultravist 370; Bayer Schering Pharma, Germany) injected through an 18-gauge intravenous antecubital catheter. A triphasic protocol was applied for contrast medium administration: 30 mL of contrast material was administered at a rate of 4.5 mL/sec, 60 mL of contrast material was administered at a rate of 2.5 mL/sec, and 30 mL of sodium chloride was administered at a rate of 2.5 mL/sec. With use of the bolus triggering technique (Care Bolus; Siemens, Forchheim, Germany), CT data acquisition was started automatically after contrast medium injection, as soon as an attenuation value of 160 HU was reached in a 20-mm-diameter region of interest placed in the ascending aorta.
No episodes of adverse allergic reactions or extravasation occurred. CT data acquisition and bolus timing procedures were successfully completed in all patients.
Image reconstruction
In group 2 and 3 ECG pulsing for radiation dose reduction was used in all retrospectively ECG-gated cCTA protocols, as previously recommended [16]: with mean heart rates below 60 bpm, full tube current was applied from 60% to 70%, at 61–70 bpm from 60% to 80%, at 71–80 bpm from 55% to 80%, and with heart rates above 80 bpm from 30% to 80% of the RR-interval. Data of helical cCTA were reconstructed with a slice thickness of 0.75 mm, a reconstruction increment of 0.4 mm, and using a soft tissue convolution kernel (B26f) and an algorithm for automatic selection of the cardiac phase with the least motion for image reconstruction (Best Phase, Siemens, Forchheim, Germany). If necessary, additional reconstructions were performed in 5% steps within the full tube current window, and those reconstruction datasets obtaining least motion artefacts were stored for further analysis. In group 3 by default, three image series for every recon job are reconstructed: one series for the low kV (100 kV) data, one for the high kV (140 kV) data, and a mixed series (M_0.3) combining the high contrast of the low kV and the resolution of the high kV series in a ration of 30%:70% to generate a similar noise and image impression like of a regular 120 kV dataset. All measurements and image evaluations in group 3 were performed in this mixed series (M_0.3).
In group 1 reconstruction parameters were: kernel B35 (a medium soft-tissue kernel), 1.0-mm effective slice thickness and 0.5-mm increment. The adaptive cardiac volume (ACV) technique, which is a standard software package provided with the Sensation 16 cardiac CT system, served as reconstruction algorithm. Heart rates of up to ≤72 bpm ACV allow for single-segmental reconstruction, and heart rates >72 bpm for two segment reconstruction of each 1.00-mm slice resolution for single-segment-reconstruction constantly was 210 ms, for two-segment reconstruction it ranged between 210 and 105 ms, subject to the heart rate [17].
Estimation of the CT radiation dose
For estimating the radiation dose for the different protocols, dose-length-product (DLP) and volume computed tomography dose index (CTDIvol) were recorded as previously shown [18]. The effective dose (ED [mSv]) of cCTA was derived from the product of the dose-length product and a conversion coefficient for the chest according to a method proposed by the European Working Group for Guidelines on Quality Criteria in CT and Huda et al. [19]. The applied conversion coefficient (k = 0.017 mSvmGy−1 cm−1) was averaged between men and women using Monte Carlo simulations.
As body weight was documented only for the minority of patients in the electronical patient file system of the hospital, the two biggest axial thoracic diameters (right-left, anterior-posterior) from skin surface to skin surface were measured in each patient to compare the two groups in terms of body habitus.
Evaluation of subjective image quality
The coronary artery tree was subdivided into 15 segments according to the scheme proposed by the American Heart Association [20]. Two observers (RWB, JMK) with an experience of 4 and 4.5 years in reading cCTA classified separately image quality of each coronary artery segment using a 4 point rating scale from 1 to 4 (1 excellent image quality; 2, acceptable image quality, not compromising diagnostic image quality; 3, poor image quality for single coronary segments; 4, non-diagnostic) on a commercially available workstation (Siemens, Leonardo Workstation, Siemens, Germany). The criteria for image quality rating were the occurrence of motion and streak artifacts, subjective image noise and visualisation of the anatomical structures. Segments were included with a diameter of at least 1 mm at their origin. Vessel segments distal to occlusions and stented segments were excluded from analysis. The two observers were blinded to the scanner-technique and to the patient’s clinical data.
Assessment of objective image quality
To obtain objective parameters of image quality of the proximal coronary arteries, image noise, attenuation, and contrast of the proximal coronary arteries, ascending aorta as well as signal-to-noise ratio (SNR) and contrast-to-noise ratio (CNR) were determined for each CT study in regions of interest (ROI) technique. To ensure consistency all measurements performed in the ROI technique were performed three times and the mean values were calculated. Image noise (IN) was defined as the standard deviation of air measured in a region of interest (ROI) presternally in front of the patient with a diameter of 3 cm. Attenuation within the lumen of the proximal coronary arteries was measured by placing the ROIs centrally in the LM, LAD, LCX, and RCA. The attenuation within the lumen of the ascending aorta was measured by placing a ROI approximately 3 cm cranial of the aortic valve. The sizes of the ROIs were chosen as large as possible without including parts of the vessel wall. The attenuation of the cardiac septum was measured in the central part of the septum with a ROI drawn as large as possible. Based on these measurements, signal-to-noise ratio (SNR) and contrast-to-noise ratio (CNR) were determined in both groups according to the following equations:
-
(a)
\( {\hbox{SNR}} = {\hbox{AAscending aorta}}/{\hbox{IN}} \); and
-
(b)
\( {\hbox{CNR}} = \left( {{\hbox{AAscending}}\,{\hbox{aorta}} - {\hbox{Aseptum}}} \right)/{\hbox{IN}}{.} \)
Statistical analysis
Analyses were performed computer-based with dedicated software (BiAS 9.02, Epsilon, Frankfurt, Germany). Patient age, attenuation values, SNR, CNR, DLP, CTDIvol, and ED are expressed as mean values ± standard deviations. We tested continuous variables for normal distribution using the Kolmogoroff-Smirnoff-Lilliefors test, corrected according to Dallal-Wilkinson as appropriate. Weighted kappa statistics were calculated for interobserver agreements for the subjective image quality rating and were interpreted by the guidelines of Landis and Koch [21]. Statistical significance was investigated with the student t test for unpaired samples, if values followed a normal distribution. Otherwise, we applied the U test according to Wilcoxon-Mann-Whitney. For further assessment of radiation dose, 95% confidence intervals were calculated as differences between median of ED in the groups. Here, non-inferiority was tested at a one-sided significance level of 2.5%. In all other cases, we considered a p-value of less than 5% to be statistically significant and used two-sided tests.
Results
Patient characteristics
The patient characteristics are summarized in Table 1. There was no significant difference in age, heart rate, and gender distribution among the three patient subgroups. Dose parameters (CTDIvol and DLP) were available in the patient protocols of all examinations. Mean thorax diameters as measure of body habitus did also not differ significantly (vertical: group 1 = 26.6 ± 2.6 cm; group 2 = 24.52 ± 3.5 cm; group 3 = 26.2 ± 3.1; horizontal: group 1 = 34.8 ± 4.4 cm; group 2 = 33.9 ± 4.2 cm; group 3 = 35.2 ± 4.0 cm; (p > 0.27)). Therefore, further analysis and comparison of radiation exposure was considered feasible and valid.
Comparison of subjective image quality
A total of 3,060 coronary artery segments with a vessel diameter of at least 1.5 mm were accessible for evaluation. An overall diagnostic image quality was found in 94.99% of all segments (2,907), while 5.01% of all segments (153) were non-evaluative (Table 3). The interobserver agreement for subjective image quality was excellent (kappa = 0.93). There were significant differences in the rate of non-diagnostic segments between DECT vs. 16- slice CT (p < 0.01; 18 vs. 123 segments) and DSCT vs. 16- slice CT (p < 0.01; 10 vs. 123 segment, Fig. 1). We observed no significant difference for diagnostic image quality between DECT vs. DSCT (p = 0.10; 18 vs.10 segments).
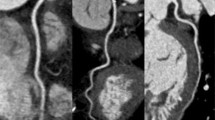
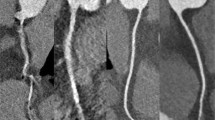
Image examples of the right coronary artery (RCA) in a curved-planar reconstruction in representative coronary CT angiography studies of group 1–3. (a) 58-year-old man from group 1 with an average heart rate of 60 bpm examined with retrospectively ECG gated 16-slice CT (estimated radiation dose 11,8mSV). Image quality was considered diagnostic in all segments of the RCA. (b) 64-year-old woman from group 2 with an average heart rate of 65 bpm examined with retrospectively ECG gated dual source CT. Image quality was diagnostic in all segments and radiation dose estimate was 9,6 mSv. (c) 58-year-old woman from group 3 with an average heart rate of 62 bpm examined with retrospectively ECG-triggered dual source CT in dual energy mode. Estimated radiation dose was 4.6 mSv and image quality was diagnostic in all segments
Comparison of objective image quality
The average attenuation of contrast medium in the ascending aorta, in the proximal LAD/RCA is displayed in Table 4.
The image noise (i. e. the standard deviation of the attenuation of air anterior to the sternum) was significantly lower for Group 2 DSCT (mean: 20.2 HU +/− 18.4) compared with group 1 (16-slice CT mean: 23.9 HU +/− 17) and group 3 (DECT mean: 29 HU +/− 21); differences between the 16-slice CT and the DECT were insignificant (p = 0.93) (Table 4, Fig. 1). The CNR in the RCA was significantly (p < 0.01) different in all groups (Table 4). There was no significant difference in CNR of the LCA between group 2 and group 3. The SNR was significantly higher in group 2 compared to group 1 and group 3. However, differences between group 1 and group 3 were insignificant (Table 4).
Radiation dose
The radiation dose parameters for the different groups are summarized in Table 3. The mean estimated radiation dose of all patients investigated on a DSCT system was 9.8 ± 4.77 mSv. The mean estimated radiation dose was 12.00 ± 3.59 mSv for 16 - slice MDCT and 4.54 ± 1.87 mSv for DECT. Significant differences were found between 16-slice CT vs. DSCT (p < 0.01), 16-slice CT vs. DECT (p < 0.01) and DECT vs. DSCT (p < 0.01).
Discussion
A variety of techniques have focused on reducing the radiation dose delivered during cCTA by modulating the tube current [16, 18]. However this conventional ECG pulsing algorithm was sensitive to arrhythmia or ectopic heart beats and could let to a mismatch of the interval of the full tube current and the desired reconstruction interval and thus to image quality degradation [16]. Modern ECG-pulsing algorithms are able to detect arrhythmia and automatically switch off the ECG pulsing during the ectopic heart beats. DSCT with an improved temporal resolution of 83 ms allows one to increase the table pitch to 0.5 at elevated heart rates. According to a study of phantom measurements, this decrease in scan time translates into linear decrease in patient dose [22]. Recently, the feasibility of applying ECG pulsing has been shown for DSCT coronary angiography and recommendations have been made for the dynamic selection of the ECG-pulsing window width in relation to the patients heart rate [16]. Following these recommendations, Stolzmann et al. reported that despite using wider pulsing windows at higher heart rates, the radiation dose of DSCT decreased as the heart rate increased. Additionally, different studies reported radiation doses between 2.1 and 4.2 mSv using prospective triggering [23–25]. However this technique is only recommended in patients at lower and stable heart rates and with a fairly low body weight [23, 26].
Another approach to lower radiation dose was reported recently by Leschka et al. obtaining a 25% reduction of radiation dose when lowering tube voltage from 120 kV to 100 kV [27]. Other studies using low radiation protocols for DSCT coronary angiography found similar results [28–30] and on the basis of theoretical considerations a dose reduction of approximately 40% can be expected [31]. However, one disadvantage of CT with lower tube voltage is the parallel increase in image noise [32, 33]. Hausleiter et al., Rixe et al., and Klass et al. have investigated image quality and radiation dose in different cCTA protocols with 16−, 64−, DSCT and 256- slice CT systems. Their data shows an increase in image noise in cCTA studies obtained at 100 kV in comparison to the standard 120 kV protocol [28, 34, 35]. Most recently a promising new technique of high pitch spiral acquisition in coronary CT angiography was introduced and showed dose levels around 0,97–2.1 mSv in the initial experience [36, 37].
In our study, we tested DECT coronary angiography consistent of a DSCT, operated with two different tube currents and two different tube voltages. Since DECT uses on tube A 140KV and on tube B 100KV it may count as a semi low voltage protocol. We observed an increase of image noise at DECT, when compared to DSCT. This effect of DECT was reported earlier by Johnson et al. [7] and is due to cross scatter radiation. However, this increased image noise (25 ± 5.0 HU) seems similar to the image noise observed by Leschka et al. using low-kV protocols (25.8 ± 3.0 HU). When we compare the contrast to noise ratio between DECT and the two other CT techniques investigated in this study we observed a higher CNR using DECT especially in the RCA, what may be caused by the low – kV dataset used in the DECT reconstruction algorithm. This phenomenon has also been shown to be present in low - kV protocols and is caused by an increase in the photoelectric effect at lower tube voltages, particularly in examinations of structures with a high anatomic number, such as iodinated contrast material [29]. As one can switch the mixture of the acquired 100 kV and 140 kV dataset while the assessment of the dataset it is possible using DECT to increase the attenuation of the contrast material in the coronary arteries by adding more of the 100 kV dataset to the mixture. This effect of DECT of the coronary arteries might be used to reduce the full amount of the applicated contrast medium as earlier proposed for chest and abdominal CT [32, 38]. Another technique to lower the amount of injected contrast material and to enhance the attenuation of the dedicated anatomical structures is the use of higher iodine concentrations of the injected contrast material as reported earlier by Rist et al. and was applied in our study in group 2 and 3, accordingly [39].
When we compare our results to the dose applied during prospective ECG-triggered cCTA (or “step-and-shoot” technique) we do not observe significantly more dose in DECT compared to the results Alkadhi et al. reported tailoring different scanning protocols in DSCT [26]. However, when prospective ECG-triggering is used data is obtained during only a short and predefined data acquisition window that usually is placed in mid-diastole. Therefore, no information on the global and regional ventricular function or valvular function can be obtained [16, 40, 41]. In contrast using DECT in every patient a full functional dataset was obtained and documented in the clinical report.
Our study has several limitations. Firstly, we did not record patient weight. To compensate for this potential limitation, we measured vertical and horizontal diameter of the thorax as parameters indicative of patient body habitus, instead. Thorax diameters, however, could be more relevant concerning radiation dose, as CTDI calibration of CT systems is usually performed with phantoms of defined diameters. However, we compared dose and image quality using the patients’ data of the last 68 patients that were investigated using the different CT techniques and whose data was saved in PACS at our institution. Doing this, we tried to avoid selection bias of the consecutive patients due to the retrospective nature of our study. No patient of the selected groups was excluded. Another limitation of this study is that different reconstruction algorithms and spatial resolutions were used in the different acquisition protocols. However, these were the clinical protocols of the different CT systems according to the literature and recommended by the vendor. A further limitation is that we did not evaluate the possibility of a prospective ECG-triggered protocol in DECT. However, as DECT is a new CT technique further research will have to show the possibilities of dose reduction using DECT.
In conclusion, using coronary DECT angiography does deliver significantly less dose than regular DSCT or single source single energy cCTA in a clinical routine setting. In addition DECT allows acquiring potential gain of information about myocardial ischemia without any increase in patient dose.
References
Schoepf UJ, Zwerner PL, Savino G, Herzog C, Kerl JM, Costello P (2007) Coronary CT angiography. Radiology 244:48–63
Achenbach S (2006) Computed tomography coronary angiography. J Am Coll Cardiol 48:1919–1928
Budoff MJ, Achenbach S, Blumenthal RS et al (2006) Assessment of coronary artery disease by cardiac computed tomography: a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation 114:1761–1791
Hendel RC, Patel MR, Kramer CM et al (2006) ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR 2006 appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American College of Radiology, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, American Society of Nuclear Cardiology, North American Society for Cardiac Imaging, Society for Cardiovascular Angiography and Interventions, and Society of Interventional Radiology. J Am Coll Cardiol 48:1475–1497
Heyer CM, Mohr PS, Lemburg SP, Peters SA, Nicolas V (2007) Image quality and radiation exposure at pulmonary CT angiography with 100- or 120-kVp protocol: prospective randomized study. Radiology 245:577–583
Fink C, Johnson TR, Michaely HJ et al (2008) Dual-energy CT angiography of the lung in patients with suspected pulmonary embolism: initial results. Rofo 180:879–883
Johnson TR, Krauss B, Sedlmair M et al (2007) Material differentiation by dual energy CT: initial experience. Eur Radiol 17:1510–1517
Marin D, Nelson RC, Schindera ST et al (2010) Low-tube-voltage, high-tube-current multidetector abdominal CT: improved image quality and decreased radiation dose with adaptive statistical iterative reconstruction algorithm—initial clinical experience. Radiology 254:145–153
Waaijer A, Prokop M, Velthuis BK, Bakker CJ, de Kort GA, van Leeuwen MS (2007) Circle of Willis at CT angiography: dose reduction and image quality-reducing tube voltage and increasing tube current settings. Radiology 242:832–839
Sahani DV, Kalva SP, Hahn PF, Saini S (2007) 16-MDCT angiography in living kidney donors at various tube potentials: impact on image quality and radiation dose. AJR Am J Roentgenol 188:115–120
Schindera ST, Nelson RC, Toth TL et al (2008) Effect of patient size on radiation dose for abdominal MDCT with automatic tube current modulation: phantom study. AJR Am J Roentgenol 190:W100–W105
Ruzsics B, Lee H, Powers ER, Flohr TG, Costello P, Schoepf UJ (2008) Images in cardiovascular medicine. Myocardial ischemia diagnosed by dual-energy computed tomography: correlation with single-photon emission computed tomography. Circulation 117:1244–1245
Ruzsics B, Lee H, Zwerner PL, Gebregziabher M, Costello P, Schoepf UJ (2008) Dual-energy CT of the heart for diagnosing coronary artery stenosis and myocardial ischemia-initial experience. Eur Radiol 18:2414–2424
Yates SJ, Pike LC, Goldstone KE (2004) Effect of multislice scanners on patient dose from routine CT examinations in East Anglia. Br J Radiol 77:472–478
Hausleiter J, Meyer T, Hermann F et al (2009) Estimated radiation dose associated with cardiac CT angiography. JAMA 301:500–507
Leschka S, Scheffel H, Desbiolles L et al (2007) Image quality and reconstruction intervals of Dual-Source CT coronary angiography: recommendations for ECG-Pulsing Windowing. Invest Radiol 42:543–549
Flohr T, Ohnesorge B (2001) Heart rate adaptive optimization of spatial and temporal resolution for electrocardiogram-gated multislice spiral CT of the heart. J Comput Assist Tomogr 25:907–923
Stolzmann P, Scheffel H, Schertler T et al (2008) Radiation dose estimates in dual-source computed tomography coronary angiography. Eur Radiol 18:592–599
Huda W, Ogden KM, Khorasani MR (2008) Converting dose-length product to effective dose at CT. Radiology 248:995–1003
Austen WG, Edwards JE, Frye RL et al (1975) A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation 51(4 Suppl):5–40
Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174
McCollough CH, Primak AN, Saba O et al (2007) Dose performance of a 64-channel dual-source CT scanner. Radiology 243:775–784
Husmann L, Valenta I, Gaemperli O et al (2008) Feasibility of low-dose coronary CT angiography: first experience with prospective ECG-gating. Eur Heart J 29:191–197
Shuman WP, Branch KR, May JM et al (2008) Prospective versus retrospective ECG gating for 64-detector CT of the coronary arteries: comparison of image quality and patient radiation dose. Radiology 248:431–437
Hirai N, Horiguchi J, Fujioka C et al (2008) Prospective versus retrospective ECG-gated 64-detector coronary CT angiography: assessment of image quality, stenosis, and radiation dose. Radiology 248:424–430
Alkadhi H, Stolzmann P, Scheffel H et al (2008) Radiation dose of cardiac dual-source CT: the effect of tailoring the protocol to patient-specific parameters. Eur J Radiol 68:385–391
Leschka S, Stolzmann P, Schmid FT et al (2008) Low kilovoltage cardiac dual-source CT: attenuation, noise, and radiation dose. Eur Radiol 18:1809–1817
Hausleiter J, Meyer T, Hadamitzky M et al (2006) Radiation dose estimates from cardiac multislice computed tomography in daily practice: impact of different scanning protocols on effective dose estimates. Circulation 113:1305–1310
Pflederer T, Rudofsky L, Ropers D et al (2009) Image quality in a low radiation exposure protocol for retrospectively ECG-gated coronary CT angiography. AJR Am J Roentgenol 192:1045–1050
Stolzmann P, Donati OF, Scheffel H et al (2010) Low-dose CT coronary angiography for the prediction of myocardial ischaemia. Eur Radiol 20:56–64
Huda W, Scalzetti EM, Levin G (2000) Technique factors and image quality as functions of patient weight at abdominal CT. Radiology 217:430–435
Nakayama Y, Awai K, Funama Y et al (2005) Abdominal CT with low tube voltage: preliminary observations about radiation dose, contrast enhancement, image quality, and noise. Radiology 237:945–951
Boone JM, Geraghty EM, Seibert JA, Wootton-Gorges SL (2003) Dose reduction in pediatric CT: a rational approach. Radiology 228:352–360
Rixe J, Conradi G, Rolf A et al (2009) Radiation dose exposure of computed tomography coronary angiography: comparison of dual-source, 16-slice and 64-slice CT. Heart 95:1337–1342
Klass O, Jeltsch M, Feuerlein S et al (2009) Prospectively gated axial CT coronary angiography: preliminary experiences with a novel low-dose technique. Eur Radiol 19:829–836
Lell M, Marwan M, Schepis T et al (2009) Prospectively ECG-triggered high-pitch spiral acquisition for coronary CT angiography using dual source CT: technique and initial experience. Eur Radiol 19:2576–2583
Ertel D, Lell MM, Harig F, Flohr T, Schmidt B, Kalender WA (2009) Cardiac spiral dual-source CT with high pitch: a feasibility study. Eur Radiol 19:2357–2362
Sigal-Cinqualbre AB, Hennequin R, Abada HT, Chen X, Paul JF (2004) Low-kilovoltage multi-detector row chest CT in adults: feasibility and effect on image quality and iodine dose. Radiology 231:169–174
Rist C, Nikolaou K, Kirchin MA et al (2006) Contrast bolus optimization for cardiac 16-slice computed tomography: comparison of contrast medium formulations containing 300 and 400 milligrams of iodine per milliliter. Invest Radiol 41:460–467
Juergens KU, Fischbach R (2006) Left ventricular function studied with MDCT. Eur Radiol 16:342–357
Alkadhi H, Desbiolles L, Husmann L et al (2007) Aortic regurgitation: assessment with 64-section CT. Radiology 245:111–121
Acknowledgement
R.W. Berner is a research consultant of Siemens AG and a participant in the Siemens speaker’s bureau.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kerl, J.M., Bauer, R.W., Maurer, T.B. et al. Dose levels at coronary CT angiography—a comparison of Dual Energy-, Dual Source- and 16-slice CT. Eur Radiol 21, 530–537 (2011). https://doi.org/10.1007/s00330-010-1954-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-010-1954-9