Abstract
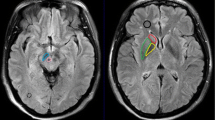
The influence of iron deposits on T2 values and the content of metabolites in the brain of three patients with DNA proved pantothenate kinase-associated neurodegeneration (PKAN, formerly Hallervorden-Spatz syndrome) was studied. An eye-of-the-tiger sign, a typical MR finding for PKAN, was observed in two patients with the same mutation. A hypointensive lesion in a whole globus pallidus was observed in the third patient with the additional mutation. T2 values in the globus pallidus of the patients were about 40% shorter than in controls (71/48 ms in controls vs. patients), which corresponds to the increase of Fe concentration based on the ferritin basis from 17 mg for controls to 48 mg (100 g wet brain weight) in PKAN patients. 1H MR spectroscopy (MRS) has mainly been used to describe neuronal damage represented by decreased NAA (6.4 mmol vs. 9 mmol) and Cr/PCr (7.0 mmol vs. 9.8 mmol) concentrations in the basal ganglia region of the patient group to controls; MRS is much more case-sensitive and describes individual development of the disease as demonstrated in the difference between the spectra of typical PKAN patients (1, 2), and the patient (3) with atypical PKAN development. Any significant changes of metabolite concentration with the exception glutamine, glutamate and GABA were found in the white matter.




Similar content being viewed by others
References
Hayflick SJ (2003) Pantothenate kinase-associated neurodegeneration (formerly Hallervorden-Spatz syndrome). J Neurol Sci 207:106–107
Zhou B, Westaway SK, Levinson B, Johnson MA, Gitschier J, Hayflick SJ (2001) A novel pantothenate kinase gene (PANK2) is defective in Hallervorden-Spatz syndrome. Nat Genet 28(4):345–349
Vogl T, Bauer M, Seiderer M, Rath M (1984) Nuclear magnetic resonance in the Hallervorden-Spatz syndrome. Digitale Bilddiagn 4(2):66–68
Littrup PJ, Gebarski SS (1985) MR imaging of Hallervorden-Spatz disease. J Comput Assist Tomogr 9(3):491–493
Pedespan JM, Fontan D, Castell JF, Langlade P, Guillard JM (1993) Contribution of nuclear magnetic imaging in the diagnosis of Hallervorden-Spatz syndrome. Arch Fr Pediatr 50(1):35–37
Hayflick SJ, Westaway SK, Levinson B, Zhou B, Johnson MA, Ching KH, Gitschier J (2003) Genetic, clinical, and radiographic delineation of Hallervorden-Spatz syndrome. N Engl J Med 348(1):33–40
Perry TL, Norman MG, Yong VW, Whiting S, Crichton JU, Hansen S, Kish SJ (1985) Hallervorden-Spatz disease: cysteine accumulation and cysteine dioxygenase deficiency in the globus pallidus. Ann Neurol 18:482–489
Rouault TA (2001) Iron on the brain. Nat Genet 28(4):299–300
Vymazal J, Brooks RA, Patronas N, Hajek M, Bulte JWM, Di Chiro G (1995) Magnetic resonance imaging of brain iron in health and disease. J Neurol Sci 134(Suppl):19–26
Roy CN, Andrews NC (2001) Recent advances in disorders of iron metabolism: mutation, mechanism and modifiers. Hum Mol Genet 10:2181–2186
Vymazal J, Brooks RA, Zak O, McRill C, Shen C, Di Chiro G (1992) T1 and T2 of ferritin at different field strengths: effect on MRI. Magn Reson Med 27(2):368–374
Bulte JWM, Vymazal J, Brooks RA, Pierpaoli C, Frank JA (1992) Frequency dependence of MR relaxation times. II. Iron oxides. J Magn Reson Imaging 3:641–648
Brooks RA, Vymazal J, Bulte JWM, Baumgarner CD, Tran V (1995) Comparison of T2 relaxation in blood, brain and ferritin. J Magn Reson Imaging 5(4):446–450
Allkemper T, Schwindt W, Maintz D, Heindel W, Tombach B (2004) Sensitivity of T2-weighted FSE sequences towards physiological iron depositions in normal brains at 1.5 and 3.0T. Eur Radiol 14:1000–1004
Schenker C, Meier D, Wichmann W, Boesiger P, Valavanis A (1993) Age distribution and iron dependency of the T2 relaxation time in the globus pallidus and putamen. Neuroradiology 35(2):119–124
Dezortová M, Hájek M, Tintěra J, Hejcmanová L, Syková E (2001) MR in phenylketonuria-related brain lesions. Acta Radiol 42(5):459–466
Herynek V, Babis M, Trunecka P, Filip K, Vymazal J, Dezortova M, Hajek M (2001) Chronic liver disease: relaxometry in the brain after liver transplantation. MAGMA 12(1):10–15
Starcuk Z, Starcukova J (2003) Determination of conficence region of model parameters in mono- and biexponential fitting of relaxation curves. Proc Int Soc Mag Reson Med 11:1102
Hallgren B, Sourander P (1958) The effect of age on the non-haemin iron in the human brain. J Neurochem 3:41–51
Provencher SW (1993) Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 30:672–679
Hájek M, Burian M, Dezortová M (2000) Application of LCModel for quality control and quantitative in vivo 1H MR spectroscopy by short echo time STEAM sequence. MAGMA 10:6–17
Jírů F, Dezortová M, Burian M, Hájek M (2003) The role of relaxation time corrections for the evaluation of long and short echo time 1H MR spectra of the hippocampus by NUMARIS and LCModel techniques. MAGMA 16:135–143
Sener RN (2003) Pantothenate kinase-associated neurodegeneration: MR imaging, proton MR spectroscopy, and diffusion MR imaging findings. Am J Neuroradiol 24:1690–1693
Dooling EC, Schoene WC, Richardson EP Jr (1974) Hallervorden-Spatz syndrome. Arch Neurol 30:70–83
Vakili S, Drew AL, Von Schuching S, Becker D, Zeman W (1977) Hallervorden-Spatz syndrome. Arch Neurol 34:729–738
Voelkl A, Ule G (1972) Spurenelementen im menschlichen Gehirn:Altersabhaengigkeit der Eisenkoncentration in 13 verschiedenen Hirnregionen. J Neurol 202:331–338
Vymazal J, Hajek M, Patronas N, Giedd JN, Bulte JWM, Baumgarner BS, Tran V, Brooks RA (1995) The quantitative relation between T1-weighted and T2-weighted MRI of normal gray matter and iron concentration. J Magn Reson Imaging 5:554–560
Dobson J (2001) Nanoscale biogenic iron oxides and neurodegenerative disease. FEBS Lett 496:1–5
Koga H, Kondo A, Kimura T, Takamatsu J (1998) Familial multiple system degeneration associated with iron deposition: determination of subcellular localization of elementary iron by energy-dispersive X-ray analysis. Clin Neuropathol 17:35–40
Furia TE (2001) CRC handbook of food additives, 2nd edn. Stability constants (log K1) of various metal chelates (web compilation-latest revision May 28, 2001). CRC, Boca Raton
Vymazal J, Bulte JW, Frank JA, Di Chiro G, Brooks RA (1993) Frequency dependence of MR relaxation times. I. Paramagnetic ions. J Magn Reson Imaging 3:637–640
Atamna H, Walter PB, Ames BN (2002) The role of heme and iron-sulfur clusters in mitochondrial biogenesis, maintenance, and decay with age. Arch Biochem Biophys 397:345–353
Kress GJ, Dineley KE, Reynolds IJ (2002) The relationship between intracellular free iron and cell injury in cultured neurons, astrocytes, and oligodendrocytes. J Neurosci 22:5848–5855
Acknowledgment
The study was supported by grant projects: Grant Agency of Czech Republic No. 304/03/1189 and CEZ:L17/98:00023001, Czech Republic.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hájek, M., Adamovičová, M., Herynek, V. et al. MR relaxometry and 1H MR spectroscopy for the determination of iron and metabolite concentrations in PKAN patients. Eur Radiol 15, 1060–1068 (2005). https://doi.org/10.1007/s00330-004-2553-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-004-2553-4