Abstract
Relatively little is known about the distribution of fish in deep water (>200 m) in the Beaufort Sea. Data collected by an Acoustic Doppler Current Profiler operated in the Chukchi and Beaufort seas in summer were examined for evidence of fish biomass detections between 18 and 400 m. The presence of fish in waters between 1 and 30 m was explored opportunistically with a non-scientific echo sounder. Evaluation of findings was enhanced by measurements of water column properties (temperature, salinity, fluorescence and transmissivity). Relatively small shoals of fish were detected on the Chukchi shelf and eastern Chukchi shelf break, and also on the Alaskan and Canadian Beaufort shelves in the upper 20 m (T = 2–5°C). Much larger shoals (putative polar cod) were detected within Atlantic Water along the Beaufort continental slope (250–350 m) and near the bottom of Barrow and Mackenzie canyons, where temperatures were above 0°C. A warm-water plume of Alaska Coastal Current water with high concentrations of phytoplankton, zooplankton, and fish was found extending along the shelf 300 km eastward of Barrow Canyon. In contrast to the warm surface and Atlantic Water layers, very few fish were found in colder, intermediate depth Pacific-origin water between them. The large biomass of fish in the Atlantic Water along the continental slope of the Chukchi and Beaufort seas represents previously undescribed polar cod habitat. It has important implications with regard to considerations of resource development in this area as well as understanding impacts of climate change.
Similar content being viewed by others
Introduction
Changes in the cryopelagic realm of the North are increasingly obvious (Anisimov et al. 2007). Vast expanses of the Arctic Ocean’s ice cover have been thinning and shrinking (Perovich and Richter-Menge 2009), with huge ecological implications (cf. Grebmeier et al. 2006, 2010; Adger et al. 2007). Ice cover has decreased to the point that large areas may soon be navigable, at least during part of the year (Serreze et al. 2007), opening for a variety of environmental and socio-economic impacts, including the exploitation of fish resources (cf. Krauss et al. 2005). Of regional concern is the Arctic Management Area (AMA), marine waters in the US Arctic Exclusive Economic Zone of the Chukchi and Beaufort seas, extending from 3 nm off the Alaskan coast to 200 nm offshore, north of the Bering Strait westward to the US/Russia maritime boundary and eastward to the US/Canada maritime boundary. Although commercial pelagic fishing is presently prohibited in the AMA, considerable potential fishing power is nearby in the North Pacific offshore fleet. In anticipation, the North Pacific Fishery Management Council has developed a Fishery Management Plan (FMP) for the AMA (NPFMC 2009), and polar cod (Boreogadus saida) is listed as a “target species” for fisheries development despite an included prohibition against fishing for forage species. The polar cod is acknowledged to play a pivotal role in Arctic ecosystems (Bradstreet et al. 1986; Power 1997; Hopcroft et al. 2008). Lowry and Frost (1981) list it as important prey for 11 species of marine mammals, 20 species of marine birds, and four species of fish, although the last estimate is probably low. It is a true ecological keystone species in the Arctic marine food web, but knowledge of its habitat and distribution is limited. As a result, the FMP describes essential fish habitat for the species as essentially all of the AMA. Knowledge of the distribution of fish habitat is essential for effective resource management and protection. It is also key to broadening our understanding of implications of global change. We here focus on describing a preferred habitat for the adult stage of this critically important forage fish.
At a maximum size of about 0.30 m, the polar cod is a pelagic species that occupies the upper part of the water column in Arctic and sub-Arctic seas, commonly to depths of about 300 m (Scott and Scott 1988), although it has been found as deep as 930 m (ADFG 1986). Acoustical studies show that polar cod are generally found in two types of aggregations: it may occur in dense shoals (Welch et al. 1993; Benoit et al. 2008), which represent important energy stores in the Arctic food web and attract large numbers of predators (Welch et al. 1992, 1993; Crawford and Jorgenson 1996), or it can be found as non-schooling individuals (Bradstreet et al. 1986; Hop et al. 1997b). It is common in the pelagic zone, at ice edges and also the epontic zone (the area near the undersurface of ice) where it preys upon fauna in the ice algae community (Bradstreet and Cross 1982; Bradstreet et al. 1986; Crawford and Jorgenson 1990, 1993; Gradinger et al. 2005). Physical irregularities at the ice edge may represent cryptic habitat for these fish, places where they can hide from a wide variety of predators (Bradstreet and Cross 1982; Lønne and Gulliksen 1989; Moore et al. 2000; Gradinger and Bluhm 2004). Early life stages are predominantly in layers of zooplankton drifting beneath the ice and in open water (Sekerak 1982; Bradstreet et al. 1986; Crawford and Jorgenson 1990; Fortier et al. 2006). Studies in the eastern Canadian Arctic archipelago have confirmed the presence of polar cod in deeper waters (cf. Lear 1979, 1983; Crawford and Jorgenson 1996) including 300 m in Lancaster Sound (Bradstreet et al. 1986). While little is known about their occurrence in offshore Canadian waters of the Beaufort Sea, notable recent research recorded the presence of vast numbers of polar cod near the bottom (200 m) throughout the winter in frozen Franklin Bay in Amundsen Gulf (Benoit et al. 2008, 2010; Geoffroy et al. 2011).
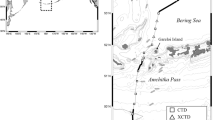
The Chukchi and Beaufort seas (Fig. 1) are physically and ecologically different (Carmack and Wassmann 2006; Piatt and Springer 2007). Because of its determinant role in polar cod habitat distribution, it is informative to consider the oceanographic complexity of the region in some detail. The broad and highly productive Chukchi Sea extends about 800 km northward from the Bering Strait to the shelf break of the Arctic Ocean (Weingartner 1997). The Beaufort Sea shelf is comparatively narrow (50–150 km) and is also much less productive due to much lower levels of available nutrients. However, the shelf break of both seas drops off rapidly into the abyssal Canada Basin of the Arctic Ocean wherein water masses of both Pacific and Atlantic origin are encountered. It is common that wind forcing at the shelf break of both seas frequently drives upwelling events that lift nutrient-rich water from the depths to the shelves (Weingartner 1997; Carmack and Kulikov 1998).
a The survey area in the Chukchi and Beaufort seas showing the different water masses that make up the water column in the area, including the Alaska Coastal Current (ACC), and Pacific summer (PSW) and winter (PWW) waters as well as the deeper Atlantic Water (AW). Also shown are the two canyons (Barrow and Mackenzie) discussed here. In the Canada Basin, the clockwise flowing Beaufort Gyre dominates the upper water column. Bathymetric contours are in meters. b and c The different water masses are also identified by the temperature (solid line in b) and salinity properties shown here. Also identified is the characteristic near surface temperature maximum (NSTM)
The upper part of the water column in the southern Beaufort Sea is predominantly made up of relatively cold and fresh Pacific water entering through the Bering Strait, while water below about 220 m is warmer (>0°C) and saline (>34) [absolute salinity] and is ultimately of Atlantic origin (called Atlantic Water or AW) (Fig. 1a). The Pacific water inflow is a combination of the relatively cool and nutrient-rich Anadyr current and the nutrient poor, but relatively warm (2–13°C) Alaska Coastal current (ACC), which is strongly influenced by freshwater runoff (salinity ~32.2) (Fig. 1a). The upper 200 m of the Canada Basin in the western Arctic Ocean is a complex layering of water masses (Fig. 1b, c) with several distinct Pacific origin water masses below a relatively fresh surface mixed layer with a near-surface temperature maximum (NSTM) at a depth of ~25 m followed by a temperature minimum suggested to be the remnant of the previous winter’s mixed layer (Jackson et al. 2010). Below these layers is a temperature maximum (>−1.0°C) that is composed of Pacific origin water that was modified in the Chukchi Sea during summer, referred to as Pacific Summer Water (PSW) by Coachman and Barnes (1961), and has a salinity of ~31–33 (Shimada et al. 2001). The deepest Pacific origin water mass is a temperature minimum found at a depth of ~150 m and a salinity of ~33.1, called Pacific Winter Water (PWW) by Coachman and Barnes (1961) and was modified in the Chukchi Sea during winter. Beneath the Pacific origin water, Atlantic Water is warm and relatively depleted in nutrients upon reaching the Beaufort Sea (McLaughlin et al. 2005).
As the ACC leaves Barrow Canyon and flows toward the Beaufort shelf, it combines with the Central Channel flow to create a strong and narrow coastal jet that follows the Beaufort Shelf topography eastward toward the Mackenzie Canyon (Pickart 2004; Winsor and Chapman 2004). There it mixes with the outflow of the Mackenzie River, which generally also flows eastward and spreads out across the Mackenzie Shelf (also called the Canadian Shelf). Between roughly mid-May to November, freshwater flow from the river—the Mackenzie plume—dominates the flow over the Mackenzie Shelf in a highly turbid surface layer about 5 m thick that overlays a brackish mixed layer from ice melt that extends to about 10–15 m (Carmack et al. 2004). The plume predominantly flows eastward into the Amundsen Gulf; its westward extent is unknown (Dunton et al. 2006).
Studies (Frost and Lowry 1983; Barber et al. 1997; Gillispie et al. 1997; Norcross et al. 2009; Rand and Logerwell 2011) show that overall pelagic fish diversity in the Chukchi Sea is higher than in western Beaufort Sea waters due to the proximity of the link to the Pacific Ocean through the Bering Strait (Wyllie-Echeverria et al. 1997). While species such as Walleye pollock (Theragra chalcogramma) and capelin (Mallotus villosus) may be encountered, in all cases by far the most common fish collected in all of these studies was the polar cod.
Early observations suggested that the polar cod is mainly associated with cold water; for example, it was reported that while larvae and fry can be found in warmer waters (4–7°C), larger fish were always encountered in water colder than 3°C (Olsen 1962). More recent work reported normal fry development between −1.5 and 3.0°C, but highest survival rates occurred between 0.5 and 3.0°C (Sakurai et al. 1998), observations supported by Fortier et al. (2006). In coastal waters, adults occasionally tolerate temperatures as warm as ~13°C, albeit they are more commonly found in waters no warmer than 6–7°C (Craig et al. 1982).
Thus, while polar cod are indeed cold loving, they use a variety of habitats, including the demersal, near-bottom region. The primary objective of this study was to identify areas and characterize specific habitats where fish (primarily polar cod) are abundant (i.e., aggregated into shoals) during some of the open water season (September–October) in largely unexplored waters along the continental shelf and slope in portions of the eastern Chukchi and southern Beaufort seas.
Methods
This study makes use of ungroundtruthed hydroacoustic data from a Teledyne RD Instruments Ocean Surveyor 76.8 kHz Acoustic Doppler Current Profiler (ADCP) operated aboard the R/V Mirai from eastern Chukchi Sea to the Mackenzie shelf—a straight line distance of roughly 1,200 km along the coast—from 7 September to 6 October 2002 (Fig. 2) during the Joint Western Arctic Climate Study (JWACS). The ADCP was operated almost continuously for the purpose of gathering data describing the current regime in the water column along the cruise track. Incidentally it also collected temporal and spatial information about the distribution and relative abundance of plankton and fish. This study, first reported in a working report with color graphics (Crawford 2009), is an a posteriori analysis of the latter data.
Distribution of relative echo strengths along the cruise track. a The eastern (dashed dot line) and western (dashed line) tracks when the 76-kHz ADCP was operating. Open circles indicate areas where the backscatter strength was category 3 (mixed plankton and fish, Table 1), and filled squares indicate areas where the backscatter strength was category 4 (fish shoals, Table 1). b and c are enlarged portions of a to reveal detail. Locations numbered from 1 to 3 and identified by arrows are discussed in the text
The ADCP system is made up of a 4-beam phased array, and to minimize contamination by the ship and its propellers only data from beam 3, pointing to the port side of the ship, were analyzed. The data are comprised of transmissions every 2 s, with 16 transmissions averaged into ensembles every 60 s. The water column was divided into 5-m-depth bins starting at a depth of 17.26 m, and data were recorded to a maximum depth of 500 m. Each data collection period lasted approximately 26 h. The ADCP collected data both when the ship was moving and while on station.
The ADCP echo amplitudes were corrected for range and frequency-dependent attenuation (WinRiver program, R.D. Instruments, Inc.). Normally reported in terms of “counts” when used for studies of ocean currents, echo amplitudes were converted to approximate backscatter intensity with the relation 1 count = 0.43 dB (S. Idle, R.D. Instruments, personal communication), recognizing that because the system was not calibrated for biomass determination the actual equivalency was between 0.41 and 0.45 dB (S. Idle, R.D. Instruments, Inc., personal communication). The target strength of adult polar cod varies between −40 and −50 dB (Crawford and Jorgenson 1996) making it technically feasible to identify echoes from individual fish in the ADCP data. However, in the present study, backscatter strength threshold values were used to identify and classify fish shoals. And by assuming that polar cod were the major contributor to the overall observed high backscatter intensity levels, the degree of fish habitat use was inferred from the observed relative fish abundance.
Based on experience gained while conducting hydroacoustic studies of polar cod elsewhere (cf. Crawford and Jorgenson 1996), a heuristic data analysis scheme was devised that was based on five backscatter signal-strength categories, or thresholds, as listed in Table 1.
A companion study (Walkusz et al. 2008) also conducted during JWACS 2002 involved collecting plankton and larval fishes with a General Oceanics 0.61 m diameter bongo net system at 33 stations along the survey line. The net sampling scheme was aided by information gathered (1–40 m) with a single beam (−3 dB beam angle 9°) 192 kHz Lowrance X-15B echo sounder operated while the ship was on station (i.e., drifting). There were two types of echoes detected with it: faint echoes suggestive of small scatterers (e.g., zooplankton and fish larvae) and more definitive prototypical fish-type echoes. The bongo/echo-sounder stations were primarily in the shelfbreak region of the eastern Chukchi Sea and the Mackenzie Shelf near the Mackenzie River. Observations with the sounder informed us of conditions extant in some of the upper 17 m, none of which was sampled by the ADCP.
Physical characteristics of the water column were obtained at 146 stations (some were also plankton sampling stations) along the ship track using a profiling Sea-Bird SBE 911, Conductivity, Temperature and Depth (CTD) system, with additional sensors for oxygen (SBE 43), fluorescence (Seapoint), and transmissivity (Chelsea/Seatech/Wetlab CStar CST-207RD).
Results
Elevated acoustic backscatter intensities indicating aggregations of biomass were contagiously distributed in the study area. In the shallow (~40 m depth) southeastern and central Chukchi Sea, biomass was distributed throughout the water column in some places (e.g., Fig. 3, #1), near the bottom in others (e.g., Fig. 3, #3), and rarely only in the upper portion of the water column. All the backscatter intensity data classified into categories 3 and 4 (Table 1) are summarized in Fig. 2.
a A cross section of the 76-kHz ADCP backscatter data collected across a portion of the SE Chukchi shelf and the length of Barrow Canyon (inset). Here, as in other places on the shelf, strong backscatter filled the shallow water column (1). In the canyon proper, which begins between 159 and 161°W, very dense aggregations of biomass were detected in the upper portion of the water column (2) and also at the bottom of the canyon (3). In b contours of salinity and temperature from 6 CTD casts along the transect have been superimposed
CTD observations of water properties along the shallow Chukchi portion of the track showed that relatively warm water (~5–6°C) from the ACC often filled the water column (Fig. 3b). Seapoint fluorescence was moderately high (>1 units, not shown) throughout the water column, indicating mixing allowed primary production to extend through the water column. On the central Chukchi shelf, acoustically recorded biomass in the flows of PSW was spread out across a wide area (Fig. 2).
The strongest backscatter signals detected during the entire study were found in Barrow Canyon, which clearly is a biological hotspot. Fed by ACC waters, very dense aggregations of biomass were detected in the canyon’s water column, centering on depths between 40 and 75 m (Fig. 3, #2). There was also a strong backscatter signal associated with fish (39–45 dB) near the bottom of the canyon at 175–250 m (Fig. 3, #3). This pattern repeated the next day when the ship crossed the canyon roughly north to south. By assuming that biomass was similarly distributed throughout the rest of the canyon, the area of biomass concentration in the Barrow Canyon region was roughly 6,500 km2. Significant biomass was detected in the canyon during September 6–8, September 14, and again on October 1, 2002, suggesting long-term presence of fish, including polar cod.
The pelagic concentrations of biomass in the canyon between 18 and 80 m were in warm (~6°C) ACC water that flowed beneath a layer of less saline, colder water (~2.5°C) at the surface to about 15 m (Fig. 4). The ACC water extended down to ~50 m and Seapoint fluorescence at the bottom of the current was high (~4 units) albeit deep (50–80 m).
Cross sections (a and c) of ADCP backscatter strength data from the transects across Barrow Canyon shown in (b). Elevated backscatter was detected between 150 and 250 m. Also shown in a are salinity contours from 10 CTD profiles along the track and a selection of temperature profiles along the same track (numbers). d Temperature (solid), salinity (dashed dot), and chlorophyll (gray) profiles at the location indicated by dashed line marked “d” in c
The currents flowing eastward through the canyon and across adjacent shelves carried the primary and secondary production offshore in a plume that extended into the Canada Basin (Fig. 2, #1). Elevated backscatter in the plume (39–45 dB, category 4, Table 1) occasionally extended to 85 m (Fig. 5a), and the backscatter signal matched the depth distribution of a warm-water mass (0–3°C) (Fig. 5b). It was a persistent and extensive feature, which was detected again 18 days later (Fig. 5c and d). There was evidence that the plume extended eastward roughly 300 km because a relatively small area of elevated acoustical backscatter intensity and Seapoint fluorescence was detected at about 72N, 146W. It too was associated with a warm-water anomaly and could have been the end of the plume emanating from the west.
a and c Backscatter strength along transects east of the mouth of Barrow Canyon (inset in c), across the plume of the currents that flow through it. There was a strong backscatter signal in the upper portion of the water column to 80 m (depth >200 m). Transect c was made 18 days after transect a. b and d CTD profiles at locations shown by dashed vertical lines in a and c [temperature (solid line), salinity (dashed dot line), and chlorophyll (gray line)]. The distances given in (a) and (c) are relative to the common point of the two transects as identified in the map insert
In addition to the repeated observations of the Barrow Canyon plume, another case of persistence was observed south of Cape Lisburne in SE Chukchi Sea (Fig. 2), where biomass was detected throughout the water column both on September 6, 2002 and again a month later (October 6, 2002).
Moving northward from Barrow canyon along the eastern edge of the Chukchi shelf, the backscatter intensity levels decreased and backscatter corresponding to category 3 (mixed plankton and fish) was more common (Fig. 2).
The southern of two survey tracks extending northeastward from the central eastern Chukchi shelf and into Canada Basin (Fig. 2) identified category 4 (fish-shoals) in the upper portion of the water column where the track crossed the shelf break. There was also a strong signal from a fish shoal deeper along the slope between about 200–375 m. Further into the Canada Basin, backscatter levels remained low and plankton biomass decreased heading away from the shelf. Along the more northern transect, no fish were detected, except for some category 3 backscatter between 20 and 50 m in a small region at the northernmost extent of the study area (Fig. 2, #2).
Markedly higher backscatter levels were nearer shore along the Alaskan Beaufort slope where fish were detected at depths between 300 and 350 m (Fig. 2, #3). The echo sounder also recorded a number of fish echoes, typical of loosely aggregated non-schooling fish in the warm (>0°C), less saline surface layer that often extended to only about 18–20 m. The occurrence of fish in this warm surface layer persisted throughout the Mackenzie region. However, the densest surface aggregation of fish (upper 15 m) was found at the shelf break near the Alaska/Canada border (Fig. 2, #4, S1), to the west of Mackenzie Bay where the water temperature was between 0 and 2°C and where very low levels of fluorescence revealed the consequence of the low nutrient content of the shelf current that is far from the Chukchi shelf (Fig. 6a). Below the warm brackish layer, water temperature dropped quickly (e.g., −1.0°C at 30 m) and fish were absent. Water temperature was also above 0°C in the upper 10 m at station S2 (Fig. 2c) on the Beaufort shelf (Fig. 6b) where fish were also common.
Temperature (solid line), salinity (dashed line), and chlorophyll concentrations (gray line) at stations S1 (a) and S2 (b) (Fig. 2c) where fish echoes were abundant in the upper 20 m at the Mackenzie shelf break and on the shelf, respectively
In addition to the fish in this region’s warm surface layer, there were also very large aggregations down deep. There was a strong backscatter signal from fish shoals near the bottom of Mackenzie Canyon (Fig. 7a) as there had been near the bottom of Barrow Canyon. The fish signal persisted further up the canyon, but the amount of backscatter diminished, suggesting fewer fish in that area. The areal extent of the elevated backscatter region at the bottom of Mackenzie Canyon (between 200 and 450 m) corresponded to an area of roughly 1,500 km2.
a Backscatter intensity data from 76-kHz ADCP from 25 September 2002 when there was a very large aggregation of biomass (1), assumed to be shoals of polar cod, along the wall of Mackenzie canyon and pelagic shoals were in the upper 50 m. b Temperature (solid), salinity (dashed dot), chlorophyll (gray), and transmissivity (dashed) profiles from inside Mackenzie Canyon
A key facet of the distribution of fish at depth in Mackenzie Canyon was that a significant portion of the biomass was where the water temperature was approaching that at the surface. Where fish were at the surface, in the mixed surface layer and brackish Mackenzie River plume, temperatures were slightly above 0°C. Beneath this water mass, temperature dropped rapidly to −1.0°C (30 m) and by 100 m it was −1.5°C. We did not detect fish in this region of the water column. Outside the canyon, along the Beaufort slope, temperature increased markedly at the lower halocline (200 m) and by 250 m (AW), where ADCP data revealed large numbers of fish, it was again around 0°C or slightly warmer. Further inside the canyon, the pattern repeated but demersal fish shoals were in shallower water (Fig. 7a). Because of upwelling, the lower halocline occurred at about 125 m and AW, with its temperatures above 0°C and where fish were, was around 200 m (Fig. 7b).
In contrast to the large amount of fish in the canyon, plankton biomass and chlorophyll concentrations were low. This stands in stark contrast to conditions in the highly productive Barrow Canyon where high nutrient levels stimulated production and chlorophyll concentrations exceeded 4 mg m−3. It is clear that the high productivity in the currents to the west of Mackenzie Canyon had been depleted of their nutrients and the currents flowing across the Mackenzie region were quite different.
Another interesting aspect of conditions in the canyon was a sharp reduction in transmissivity at the halocline at the bottom of the surface layer where the fish were congregating (Fig. 7b). Concomitantly, there was only a minor increase in Seapoint fluorescence, suggesting a layer of zooplankton and phytodetritus (marine snow) at the density boundary. Deeper in the water column (40 m), there was a slight decrease in transmissivity at the deep chlorophyll maximum, where low fluorescence (0.6 units) indicated low biomass of phytoplankton. Transmissivity remained high to the lower halocline at about 200 m where there was a small decrease that marked the transition from −1.5°C to the warmer water mass of Atlantic Water (0.5°C at 350 m). At that point, where the water reached about its warmest temperature, and where the fish were, there was a marked drop in transmissivity but no fluorescence, likely due to suspended sediments. Thus, the fish at the bottom at the mouth of the canyon were in a mildly turbid layer.
Discussion
Polar cod distributions in the eastern Chukchi Sea and along the Beaufort shelf revealed three main patterns: (1) they are found near the surface (epipelagic habitat) in northeastern Chukchi Sea and Alaskan/Canadian Beaufort shelf; (2) they are found in demersal habitat along the eastern Chukchi and Alaskan/Canadian Beaufort slope in the upper layer of Atlantic Water between 200 and 350 m; and (3) they are found in association with a large plume of pelagic biomass within a warm-water (Pacific-origin) eddy that, during this study, extended from Barrow Canyon well into the Beaufort Sea down to depths of 85 m.
Since only a small fraction of the Chukchi Sea was surveyed, it is not possible to generalize about the distribution of surface layer fish in the Chukchi region, other than to say that fish were detected in the warm water layer in the upper 20 m, and that it appears that fish—larvae, juveniles or adults—did not occur much beyond the shelf break.
Ashjian et al. (2005) also observed the plume in Barrow Canyon and determined it consisted largely of copepods, diatom chains, decaying diatoms, radiolarians, and high concentrations of marine snow (mostly organic detritus). Given that our transmissometer readings in the plume (80 percent) were only slightly less than typical readings taken elsewhere (83–85 percent), where there was only modest backscatter, we believe fish were contributing to the strong ADCP echo from this feature.
The occurrence of polar cod in warm surface waters in the western Arctic was first observed near Prudhoe Bay in 1978–1979, where polar cod were found to limit their depth distribution to a warm, low-salinity water mass T = 2–9°C; S = 6–27 (Moulton and Tarbox 1987). Our results show that polar cod occupy the warm surface layer from the coast to the edge of continental shelf, at least along some portions of the Beaufort shelf. Acoustical data also showed that polar cod were common in the warm, brackish surface layer of Mackenzie Shelf waters in waters ranging in salinities between about 15–34 and with a wide range of turbidity levels. Therefore, neither salinity nor turbidity are thought to be forcing factors affecting their distribution. We could not confirm that fish were within the 3-m-thick lens of highly turbid Mackenzie River water on top of the brackish plume because it was essentially acoustically opaque. Nevertheless, in echograms, fish appeared to be moving between the two layers. Given their euryhaline physiology, we assume that polar cod occasionally venture into at least the bottom of the turbid Mackenzie River water and occur in the brackish plume from about 3 m to 15–20 m.
The depth distribution of polar cod detected in the present study is similar to the distribution reported for eastern Canadian Arctic. Best catches during exploratory fishing with bottom trawls off northern Labrador and Baffin Island were taken between 100 and 250 m (T = −1.4–0.6°C). Off southern Labrador and Newfoundland, catches were mainly between 200 and 300 m (T = −1.2–3.6°C) (Lear 1983).
A salinity versus temperature diagram from casts along the survey tracks and the presence of polar cod acoustical backscatter. Filled dark circles are corresponding salinity and temperature values at depths and locations of polar cod backscatter. The circled numbers are identifiers for regions discussed in the text
To highlight the strong connection between polar cod presence and the differing water mass distributions, we have plotted on T/S correlation diagrams the hydrographic data obtained at stations where polar cod shoals were observed (Fig. 8). The corresponding T/S properties where fish were present have been highlighted in the figure. The warm and salty upper layer waters identified by #1 in Fig. 8 are associated with upwelling in the Barrow Canyon. Likewise, the bottom portions of Barrow and Mackenzie canyons have Atlantic-origin waters that are warmer than 0°C, and this may explain the presence of dense aggregates there.
It is notable to see that even though the temperature environment represents no real physiological barrier to polar cod, the fish are restricted in their depth distribution to the upper layer where temperatures were warmer than −1.5°C and the deeper warmer AW where temperatures were warmer than 0°C. They are confined above the T Max of AW (Fig. 8 #2) and avoid the markedly colder PWW defined by salinities between 32.5 and 34.5.
This distribution by temperature regime is similar to what was observed in the eastern Arctic. Crawford and Jorgenson (1996) found polar cod throughout the water column (130 m) where there was little temperature stratification (t = −1.3 to −0.3°C) but in stratified waters they were concentrated in the upper 20 m when it was significantly warmer (t = >2.0°C) than waters below (t ≈ −1.5°C). We suggest that in summer, the PWW offers neither optimal temperatures nor food for shoaling cod. Geoffroy et al. (2011) observed adult polar cod also avoided PWW in winter in Amundsen Gulf despite the presence of prey there. These authors reported juveniles did not display the same avoidance behavior and pursued prey into PWW in the diurnal vertical migration. Juvenile polar cod in eastern Arctic waters display the same lack of temperature preference (Crawford, personal observation), indicating this behavior is related to fish age.
Geoffroy et al. (2011) hypothesized adults stay deep to avoid predation by diving seals but this does not explain why they remain in the deep habitat in summer where they remain vulnerable to predation by beluga whales (Delphinapterus leucas). Because beluga feed primarily on polar cod (Bradstreet et al. 1986), their feeding areas indicate the location of concentrations of the fish (Harwood and Smith 2002). Aerial surveys flown in the western Arctic during September in the 1980s and 1990s yielded sightings of belugas on the outer portion of the Mackenzie Shelf and on the slope (Clarke et al. 1993; Moore et al. 2000; Richard et al. 2001). More recent studies monitoring movements of tagged belugas also showed them following the slope region (Richard et al. 2001) where we detected fish near the bottom (200–350 m). Richard et al. (2001) concluded that belugas spent much of their time offshore of the Mackenzie Shelf and in other areas to the east—some in Amundsen Gulf/Franklin Bay area and some much further to the north, such as in Viscount Melville Sound where they stayed for about 2 weeks diving repeatedly into a 500-m trench (Richard et al. 2001). We suggest they occupy these areas to prey on polar cod aggregations at depth.
Moulton and Tarbox (1987) hypothesized that polar cod remained in the warmer surface layer to maximize energy conversion during the summer feeding period. Our results support the hypothesis that the fish occupy a habitat that represents the best choice with regard to energetic efficiencies. Although their feeding efficiency is quite high, they grow very slowly at 0°C (Hop et al. 1997a); growth appears to be faster in warmer waters (Gillispie et al. 1997). Sameoto (1984) suggested that polar cod aggregate in warmer water in summer to enhance gonad development. Although male gonads start developing during this time, females do not (Hop et al. 1995) and the notion remains untested. Hop and Tonn (1998) noted that slow digestion rates exhibited by polar cod may limit their daily food intake, even when food is abundant. Given that digestion occurs more rapidly at warmer temperatures, this habitat preference may enhance energy intake. It has even been suggested that the species is metabolically cold-adapted [i.e., their physiological activity levels are higher than in other non-adapted fishes at low temperatures] (Holeton 1974), but Steffensen et al. (1994) and Hop and Graham (1995) found no evidence to support this claim. Although an energy budget has been prepared for polar cod at about 0°C (Hop et al. 1997a), detailed examinations of thermal adaptation in polar cod to slight changes in temperature (e.g., physical endowments (Lucassen et al. 2006) or the response of enzymatic processes) have yet to be done (Christiansen et al. 1997).
We know now that polar cod utilize two main water mass habitats. Although we do not know how the fish population is proportionally spread between the two, the loss of sea ice due to climate change would mainly affect the upper habitat directly. Subsequent alterations in habitat partitioning may thus affect utilization of the deep habitat too. Consequently, before fishing for polar cod is allowed in the Arctic, it is prudent to understand what this change means to them and the ecosystem that so strongly depends upon this fish.
References
ADFG (1986) Alaska habitat management guide: life histories and habitat requirements of fish and wildlife. Alaska Department of Fish and Game, Juneau
Adger et al (2007) Climate change 2007: impacts, adaptation and vulnerability. Working Group II contribution to the Intergovernmental Panel on Climate Change. Fourth assessment report summary for policymakers
Anisimov OA, Vaughan DG, Callaghan TV, Furgal C, Marchant H, Prowse TD, Vilhjálmsson H, Walsh JE (2007) Polar regions (Arctic and Antarctic). In: Parry ML et al (eds) Climate change 2007: impacts, adaptation and vulnerability. Contribution of working group II to fourth assessment report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, pp 653–685
Ashjian CJ, Gallager SM, Plourde S (2005) Transport of plankton and particles between the Chukchi and Beaufort Seas during summer 2002, described using a video plankton recorder. Deep-Sea Res II 52:3259–3280. doi:10.1016/j.dsr2.2005.10.012
Barber WE, Smith RL, Vallarino M, Meyer RM (1997) Demersal fish assemblages of the northeastern Chukchi Sea, Alaska. Fish Bull 95:195–209
Benoit D, Simard Y, Fortier L (2008) Hydroacoustic detection of large winter aggregations of Arctic cod (Boreogadus saida) at depth in ice-covered Franklin Bay (Beaufort Sea). J Geophys Res. doi:10.1029/2007JC004276
Benoit D, Simard Y, Gagné J, Geoffroy M, Fortier L (2010) From polar night to midnight sun: photoperiod, seal predation, and the diel vertical migrations of polar cod (Boreogadus saida) under landfast ice in the Arctic Ocean. Polar Biol 33:1505–1520. doi:10.1007/s00300-010-0840-x
Bradstreet MSW, Cross WE (1982) Trophic relationships at high Arctic ice edges. Arctic 35:1–12
Bradstreet MSW, Finley KJ, Sekerak AD, Griffiths WB, Evans CR, Fabijan MF, Stallard HE (1986) Aspects of the biology of Arctic cod (Boreogadus saida) and its importance in Arctic marine food chains. Can Tech Rep Fish Aquat Sci 1491
Carmack E, Kulikov Y (1998) Wind-forced upwelling and internal Kelvin wave generation in Mackenzie Canyon, Beaufort Sea. J Geophys Res 103:18, 447–18, 458
Carmack E, Wassmann P (2006) Food webs and physical–biological coupling on pan-Arctic shelves: unifying concepts and comprehensive perspectives. Prog Ocean 71:446–477
Carmack EC, Macdonald RW, Jasper S (2004) Phytoplankton productivity on the Canadian Shelf of the Beaufort Sea. Mar Ecol Prog Ser 277:37–50
Christiansen JS, Schurmann H, Karamushko LI (1997) Thermal behaviour of polar fish: a brief survey and suggestions for research. Cybium 21:262–353
Clarke JT, Moore SE, Johnson MM (1993) Observations on beluga fall migration in the Alaskan Beaufort Sea, 1982–87, and northeastern Chukchi Sea, 1982–1991. Rep Int Whal Comm 43:387–396
Coachman LK, Barnes CA (1961) The contribution of Bering Sea water to the Arctic Ocean. Arctic 14:147–161
Craig PC, Griffiths WB, Haldorson L, McElderry H (1982) Ecological studies of Arctic cod (Boreogadus saida) in Beaufort Sea coastal waters, Alaska. Can J Fish Aquat Sci 39:395–406
Crawford RE (2009) Forage fish habitat distribution near the Alaskan coastal shelf areas of the Beaufort and Chukchi seas. Final Report, PWS Oil Spill Recovery Institute, Cordova
Crawford RE, Jorgenson J (1990) Density distribution of fish in the presence of whales at the admiralty inlet landfast ice edge. Arctic 43:215–222
Crawford RE, Jorgenson J (1993) Schooling behaviour of Arctic cod in relation to drifting pack ice. Environ Biol Fishes 36:345–357
Crawford RE, Jorgenson J (1996) Quantitative studies of Arctic cod (Boreogadus saida) schools: important energy stores in the Arctic food web. Arctic 49:181–193
Dunton KH, Weingartner T, Carmack EC (2006) The nearshore western Beaufort Sea ecosystem: circulation and importance of terrestrial carbon in arctic coastal food webs. Prog Oceanogr 71:362–378
Fortier L, Sirois P, Michaud J, Barber D (2006) Survival of Arctic cod larvae (Boreogadus saida) in relation to sea ice and temperature in the Northeast Water Polynya (Greenland Sea). Can J Fish Aquat Sci 63:1608–1616. doi:10.1139/F06-064
Frost KJ, Lowry LF (1983) Demersal fishes and invertebrates trawled in the northeastern Chukchi and western Beaufort Seas, 1976–1977. NOAA Tech Rpt NMFS SSRF-764
Geoffroy M, Robert D, Darnis G, Fortier L (2011) The aggregation of polar cod (Boreogadus saida) in the deep Atlantic layer of ice-covered Amundsen Gulf (Beaufort Sea) in winter. Polar Biol. doi: 10.1007/s00300-011-1019-9
Gillispie JG, Smith RL, Barbour E, Barber WE (1997) Distribution, abundance, and growth of Arctic cod in the northeastern Chukchi Sea. In: Reynolds J (ed) Fish ecology in Arctic North America. Spec Publ Am Fish Soc 19:81–89
Gradinger RR, Bluhm BA (2004) In situ observations on the distribution and behavior of amphipods and Arctic cod (Boreogadus saida) under the sea ice of the High Arctic Canada Basin. Polar Biol 27:595–603. doi:10.1007/s00300-004-0630-4
Gradinger RR, Meiners L, Plumley G, Zhang Q, Bluhm BA (2005) Abundance and composition of the sea-ice meiofauna in off-shore pack ice of the Beaufort Gyre in summer 2002 and 2003. Polar Biol 28:171–181. doi:10.1007/s00300-004-0674-5
Grebmeier JM, Overland JE, Moore SE, Farley EV, Carmack EC, Cooper LW, Frey KE, Helle JH, McLaughlin FA, McNutt SL (2006) A major ecosystem shift in the northern Bering Sea. Science 310:1461–1464
Grebmeier JM, Moore SE, Overland JE, Frey KE, Gradinger R (2010) Biological response to recent Pacific Arctic Sea Ice retreats. Eos Trans AGU. 91 doi:10.1029/2010EO180001
Harwood LA, Smith TG (2002) Whales of the inuvialuit settlement region in Canada’s Western Arctic: an overview and outlook. Arctic 55(Supp 1):77–93
Holeton GF (1974) Metabolic cold adaptation of polar fish: fact or artefact? Physiol Zool 47:137–152
Hop H, Graham M (1995) Respiration of juvenile Arctic cod (Boreogadus saida): effects of acclimation, temperature, and food intake. Polar Biol 15:359–367
Hop H, Tonn WM (1998) Gastric evacuation rates and daily rations of Arctic cod (Boreogadus saida) at low temperatures. Polar Biol 19:293–301
Hop H, Graham M, Trudeau VL (1995) Spawning energetics of Arctic cod (Boreogadus saida) in relation to seasonal development of the ovary and plasma sex steroid levels. Can J Fish Aquat Sci 52:541–550
Hop H, Tonn WM, Welch HE (1997a) Bioenergetics of Arctic cod (Boreogadus saida) at low temperatures. Can J Fish Aquat Sci 54:1772–1784
Hop H, Welch H, Crawford RE (1997b) Population structure and feeding ecology of Arctic cod schools in the Canadian High Arctic. In: Reynolds J (ed) Fish ecology in Arctic North America. Spec Publ Am Fish Soc 19:68–79
Hopcroft R, Bluhm B, Gradinger R (eds) (2008) Arctic ocean synthesis: analysis of climate change impacts in the Chukchi and Beaufort Seas with strategies for future research. Institute of Marine Sciences, University of Alaska, Fairbanks
Jackson JM, Carmack EC, McLaughlin FA, Allen SE, Ingram RG (2010) Identification, characterization, and change of the near-surface temperature maximum in the Canada Basin, 1993–2008. J Geophys Res 115:C05021. doi:10.1029/2009JC005265
Krauss C, Myers SL, Revkin AC, Romero S (2005) As polar ice turns to water, dreams of treasure abound. The New York Times. http://www.nytimes.com/2005/10/10/science/10arctic.html. Accessed 6 Feb 2009
Lear WH (1979) Distribution, size and sexual maturity of Arctic cod (Boreogadus saida) in the northwest Atlantic during 1959–1978. CAFSAC Res Doc 79/17. 40 pp
Lear WH (1983) Arctic cod. Department of Fisheries and Oceans Underwater World webpage. http://www.dfo-mpo.gc.ca/zone/underwater_sous_marin/ArcticCod/artcod-saida_e.htm. Accessed 6 Feb 2009
Lønne OJ, Gulliksen B (1989) Size, age and diet of polar cod, Boreogadus saida (Lepechin 1773), in ice covered waters. Polar Biol 9:187–191
Lowry LF, Frost KJ (1981) Distribution, growth, and foods of Arctic cod (Boreogadus saida) in the Bering, Chukchi, and Beaufort seas. Canad Field-Natur 95:186–191
Lucassen M, Koschnick N, Eckerle LG, Pörtner H-O (2006) Mitochondrial mechanisms of cold adaptation in cod (Gadus morhua L.) populations from different climatic zones. J Exp Biol 209:2462–2471. doi:10.1242/jeb.02268
McLaughlin F, Shimada K, Carmack E, Itoh M, Nishino S (2005) The hydrography of the southern Canada Basin, 2002. Polar Biol 28:182–189
Moore SE, DeMaster DP, Dayton PK (2000) Cetacean habitat selection in the Alaskan Arctic during summer and autumn. Arctic 53:432–447
Moulton LL, Tarbox KE (1987) Analysis of Arctic cod movements in the Beaufort Sea nearshore region, 1978–1979. Arctic 40:43–49
Norcross BL, Holladay BA, Busby MS, Mier KL (2009) Demersal and larval fish assemblages in the Chukchi Sea. Deep-Sea Res II. doi:10.1016/j.dsr2.2009.08.006
NPFMC (2009) Fishery management plan for fish resources of the Arctic management area. North Pacific Fishery Management Council, Anchorage
Olsen S (1962) Observations on polar cod in the Barents Sea. ICES Conf Mtg Doc 1962/35, 3 pp
Perovich DK, Richter-Menge JA (2009) Loss of sea ice in the Arctic. Ann Rev Mar Sci 1:417–441
Piatt JF, Springer AM (2007) Marine ecoregions of Alaska. In: Spies R (ed) Long-term ecological change in the Northern Gulf of Alaska. Elsevier, Amsterdam, pp 522–526
Pickart RS (2004) Shelfbreak circulation in the Alaskan Beaufort Sea: mean structure and variability. J Geophys Res 109:C04024. doi:10.1029/2003JC001912
Power G (1997) A review of fish ecology in Arctic North America. In: Reynolds J (ed) Fish ecology in Arctic North America. Spec Publ Am Fish Soc 19:13–39
Rand KM, Logerwell EA (2011) The first demersal trawl survey of benthic fish and invertebrates in the Beaufort Sea since the late 1970s. Polar Biol 34:475–488. doi:10.1007/s00300-010-0900-2
Richard PR, Martin AR, Orr JR (2001) Summer and autumn movements of belugas of the eastern Beaufort Sea stock. Arctic 54:223–236
Sakurai Y, Ishii K, Nakatani T, Yamaguchi H, Anma G, Jin M (1998) Reproductive characteristics and effects of temperature and salinity on the development and survival of eggs and larvae of Arctic cod (Boreogadus saida). Mem Fac Fish Hokkaido Univ 45:77–89
Sameoto D (1984) Review of current information on Arctic cod (Boreogadus saida Lepechin) and bibliography. Bedford Institute of Oceanography, Dartmouth
Scott WB, Scott MG (1988) Atlantic fishes of Canada. Can Bull Fish Aquat Sci 219
Sekerak AD (1982) Young-of-the-year cod (Boreogadus) in Lancaster Sound and Western Baffin Bay. Arctic 35:75–87
Serreze MC, Holland HH, Stroeve J (2007) Perspectives on the Arctic’s shrinking sea-ice cover. Science 315:1533–1536
Shimada K, Carmack EC, Hatakayama K, Takazawa T (2001) Varieties of shallow temperature maximum waters in the western Canadian Basin of the Arctic Ocean. Geophys Res Lett 28:3441–3444
Steffensen JF, Bushnell PG, Schurmann H (1994) Oxygen consumption in four species of teleosts from Greenland: no evidence of metabolic cold adaptation. Polar Biol 14:49–54
Walkusz W, Paulic JE, Papst MH, Kwasniewski S, Chiba S, Crawford RE (2008) Zooplankton and ichthyoplankton data collected from the Chukchi and Beaufort seas during the R/V Mirai cruise, September 2002. Can Data Rpt Fish Aquat Sci 2802
Weingartner TJ (1997) A review of the physical oceanography of the northeastern Chukchi Sea. In: Reynolds J (ed) Fish ecology in Arctic North America. Spec Publ Am Fish Soc 19:40–59
Welch HE, Bergmann MA, Siferd TD, Martin KA, Curtis MF, Crawford RE, Conover RJ, Hop H (1992) Energy flow through the marine ecosystem of Lancaster Sound Region, Arctic Canada. Arctic 45:343–357
Welch HE, Crawford RE, Hop H (1993) Occurrence of Arctic cod (Boreogadus saida) schools and their vulnerability to predation in the Canadian High Arctic. Arctic 46:331–339
Winsor P, Chapman DC (2004) Pathways of Pacific water across the Chukchi Sea: a numerical model study. J Geophys Res 109:C03002. doi:10.1029/2003JC001962
Wyllie-Echeverria T, Barber WE, Wyllie-Echeverria S (1997) Water masses and transport of age-0 Arctic cod and age-0 Bering flounder into the Northeastern Chukchi Sea. In: Reynolds J (ed) Fish ecology in Arctic North America. Spec Publ Am Fish Soc 19:60–67
Acknowledgments
Data collection was funded by the Department of Fisheries and Oceans Canada. Several crewmembers of the R/V Mirai assisted with sampling. This report benefited from the thoughtful comments of Haakon Hop and two anonymous reviewers. This analysis work was funded by the Oil Spill Recovery Institute, Cordova, Alaska, as OSRI Contract 09-10-03.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Crawford, R.E., Vagle, S. & Carmack, E.C. Water mass and bathymetric characteristics of polar cod habitat along the continental shelf and slope of the Beaufort and Chukchi seas. Polar Biol 35, 179–190 (2012). https://doi.org/10.1007/s00300-011-1051-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-011-1051-9